The Advisory Committee on Vaccines of the Spanish Association of Pediatrics (CAV-AEP) updates the immunization schedule every year, taking into account epidemiological data as well as evidence on the effectiveness and efficiency of vaccines.
The present schedule includes grades of recommendation. We have graded as routine vaccinations those that the CAV-AEP believes all children should receive; as recommended those that fit the profile for universal childhood immunization and would ideally be given to all children, but that can be prioritized according to the resources available for their public funding; and as risk group vaccinations those that specifically target individuals in situations of risk. Immunization schedules tend to be dynamic and adaptable to ongoing epidemiological changes. Nevertheless, the achievement of a unified immunization schedule in all regions of Spain is a top priority for the CAV-AEP.
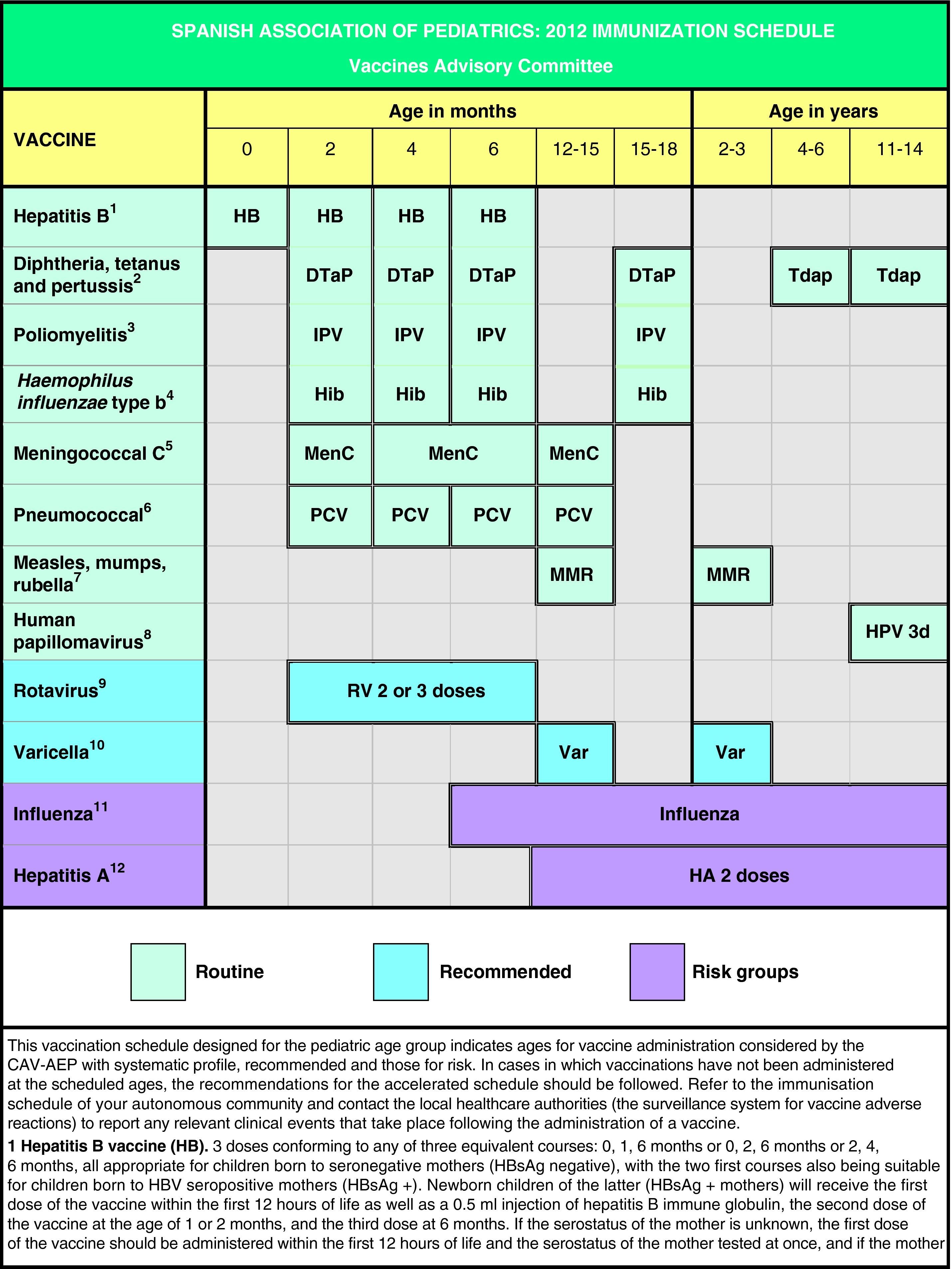
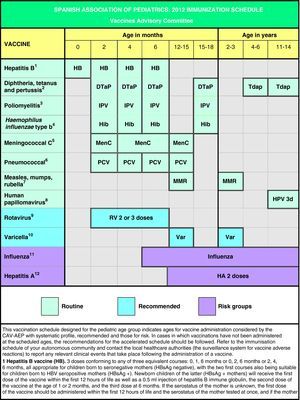
Based on the latest epidemiological trends, the main changes introduced to the schedule are the administration of the first dose of the MMR and the varicella vaccines at age 12 months (12–15 months) and the second dose at age 2–3 years, as well as the administration of the Tdap vaccine at age 4–6 years, always followed by another dose at 11–14 years of age.
The CAV-AEP believes that the coverage of vaccination against human papillomavirus in girls aged 11–14 years must increase. It reasserts its recommendation to include vaccination against pneumococcal disease in the routine immunization schedule. Universal vaccination against varicella in the second year of life is an effective strategy and therefore a desirable objective. Vaccination against rotavirus is recommended in all infants due to the morbidity and elevated healthcare burden of the virus. The Committee stresses the need to vaccinate population groups considered at risk against influenza and hepatitis A. Finally, it emphasizes the need to bring incomplete vaccinations up to date following the catch-up immunization schedule.
El Comité Asesor de Vacunas de la Asociación Española de Pediatría (CAV-AEP) actualiza anualmente el calendario de vacunaciones teniendo en cuenta tanto aspectos epidemiológicos, como de efectividad y eficiencia de las vacunas.
El presente calendario incluye grados de recomendación. Se han considerado como vacunas sistemáticas aquellas que el CAV-AEP estima que todos los niños deberían recibir; como recomendadas las que presentan un perfil de vacuna sistemática en la edad pediátrica y que es deseable que los niños reciban, pero que pueden ser priorizadas en función de los recursos para su financiación pública y dirigidas a grupos de riesgo aquellas con indicación preferente para personas en situaciones de riesgo. Los calendarios de vacunaciones tienen que ser dinámicos y adaptarse a los cambios epidemiológicos que vayan surgiendo, pero el CAV-AEP considera como objetivo prioritario la consecución de un calendario de vacunación único para toda España.
En base a los últimos cambios en la epidemiología de las enfermedades, las principales novedades propuestas en este calendario son la administración de la primera dosis de las vacunas triple vírica y varicela a los 12 meses (12–15 meses) y la segunda dosis a los 2–3 años, así como la administración de la vacuna Tdpa a los 4–6 años siempre acompañada de otra dosis a los 11–14 años.
El CAV-AEP estima que deben incrementarse las coberturas de vacunación frente al papilomavirus humano en las niñas de 11 a 14 años. Se reafirma en la recomendación de incluir la vacunación frente al neumococo en el calendario de vacunación sistemática. La vacunación universal frente a la varicela en el segundo año de vida es una estrategia efectiva y por tanto un objetivo deseable. La vacunación frente al rotavirus, dada la morbilidad y la elevada carga sanitaria, es recomendable en todos los lactantes. Se insiste en la necesidad de vacunar frente a la gripe y la hepatitis A a todos los que presenten factores de riesgo para dichas enfermedades. Finalmente, se insiste en la necesidad de actualizar las vacunaciones incompletas con las pautas de vacunación acelerada.
As has been done in previous years, the Spanish Association of Pediatrics Vaccine Advisory Committee (CAV-AEP) is updating the immunization schedule, taking into account the available evidence on the efficacy and efficiency of childhood vaccines, as well as the epidemiology of vaccine-preventable diseases in Spain.
These recommendations are addressed to pediatricians, general practitioners, nursing staff, children's relatives, and generally to all individuals who want updated information on paediatric vaccinations.
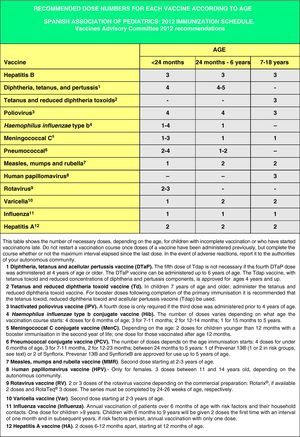
In Spain the official vaccination schedules are fully funded by the public healthcare system. For this reason, the schedules performed by this committee since 2010 include degrees of recommendation for different vaccines with the purpose of establishing priority levels for the public funding of their administration. This grading takes into account vaccine efficacy and safety data, but also the burden of disease in our environment, and, whenever possible, efficiency criteria. The pediatrician should be guided by similar considerations when advising parents with regards to the vaccines considered by this schedule but excluded from the official schedules. Fig. 1 shows the vaccination schedule recommended by the CAV-AEP for 2012, which grades immunizations into routine, recommended, and risk group vaccinations. Routine vaccinations are those that the CAV-AEP considers should be given to all Spanish children; recommended vaccinations are those that fit the profile for a universal childhood vaccination and whose administration to all children is seen as desirable, but whose priority level has to be determined according to the economic feasibility of their public funding due to issues of cost-effectiveness; and risk group vaccinations include those indicated for individuals whose environmental or personal circumstances increase their risk of contracting or having a more severe form of the disease targeted by the vaccine, or who have an underlying pathology that could be exacerbated or destabilized if they were to contract the infectious disease.
The committee continues to stress the need to ensure that routine immunizations reach every child, eliminating ethnic, territorial, social and economic disparities. One of its priority objectives is to bring the immunization schedules of immigrant children and children with incomplete vaccination up to date for the purposes of their personal protection against vaccine-preventable diseases, and also to prevent pockets of susceptible populations that could give rise to epidemic outbreaks, as happened recently with measles in Spain. The sporadic contacts that some children have with health services (emergency room, hospital admissions, pediatrician, general practitioner or nurse visits) must be used as opportunities to bring their immunization schedule up to date.
Based on the latest epidemiological changes, the main modifications introduced in relation to the recommendations issued by this committee for year 2011,1 are the following:
- -
It is recommended that the first dose of the MMR and varicella be given preferably at 12 months, although administration at 12–15 months is considered acceptable.
- -
The second doses of both MMR and varicella are recommended at 2–3 years of age, preferably at 2.
- -
The age range for the booster immunizations against group C meningococcal disease and pneumococcal disease has been set at 12–15 months.
- -
If the epidemiological circumstances call for it, a booster dose of the group C meningococcal vaccine is recommended for children that had received primary vaccination in the first year of life without a booster dose after 12 months of age.
- -
The Tdap vaccine is recommended at 4–6 years of age, always followed by another dose of Tdap at age 11–14. The recommended age has been lowered for the Tdap from 14–16 years to 11–14 years.
The CAV-AEP considers the achievement of a unified vaccination schedule a top priority in order to uphold the equity principle in preventative healthcare and to apply the rationality principle to support compliance with immunization in children whose residence moves from one autonomous community to another. At present there are no epidemiological differences in vaccine-preventable diseases among autonomous communities, with the possible exception of hepatitis A in Ceuta and Melilla, to justify the existence of different immunization schedules.2 The CAV-AEP believes that all the healthcare and political decision-making agents involved in the design of the Spanish childhood immunization schedule must make a concerted effort to this end, and continues to offer its support toward this sensible objective.
Immunization against hepatitis BImmunization against hepatitis B requires three doses that can be administered in any of the following equivalent courses: 0, 1 and 6 months; 0, 2 and 6 months; 2, 4 and 6 months. All three schedules are appropriate for children of seronegative mothers (HBsAg negative), and the first two can also be used in children of hepatitis B carrier mothers (HBsAg positive). The latter must also receive 0.5ml of specific hepatitis B immune globulin, preferably within the first 12h of life at an anatomical site different from that of the vaccine. Immunization with 4 doses of the vaccine is acceptable in autonomous communities where the monovalent vaccine against hepatitis B is given at birth if the combined hexavalent vaccine (DTaP-IPV-Hib-HB) is used for the doses at 2, 4 and 6 months of age.2 Another option in communities that implement routine vaccination of newborns is administering the hexavalent vaccine at 2 and 6 months and the pentavalent vaccine (DTaP-IPV-Hib) at 4 months.3,4
Catch-up vaccination against hepatitis B in unvaccinated older children and adolescents will follow a schedule of doses at intervals of 0, 1 and 6 months.4
Vaccination against diphtheria, tetanus and pertussis (DTaP), poliomyelitis (IPV) and Haemophilus influenzae type b (Hib)The use of combined vaccines facilitates the co-administration of several vaccines at the same time and at the same anatomical site, while reducing the number of injections and discomfort for the child, avoiding possible mistakes, shortening the duration of the administration, and simplifying the vaccination schedule. For all of these reasons, the CAV-AEP continues to recommend the use of the hexavalent vaccine (DTaP-IPV-Hib-HB) for primary vaccination at 2, 4 and 6 months.
It is possible to resort to the pentavalent preparation for economic reasons or if there is supply shortage of the hexavalent vaccine, completing the routine schedule with one or several doses of hepatitis B single component vaccine following the recommended course of vaccination. Scientific evidence gathered through extensive clinical tests supports the use of these combined vaccines, and shows no incompatibility with other immunizations nor any significant antigenic interferences.3,5,6 We must also note that the pentavalent vaccine is the best choice for booster doses at 15–18 months (fourth doses of DTaP, IPV and Hib).
In Spain, the fifth dose of the diphtheria, tetanus and pertussis vaccine at 4–6 years of age has been usually administered as DTaP (Infanrix®). However, since 2010 there has been a trend to replace it with the Tdap vaccine (which has a lower antigen load of tetanus and diphtheria), already implemented in 13 autonomous communities and the 2 autonomous cities.2 This measure has been based on the available evidence3,7–9 and on the recommendations of the Vaccination Registry Report and the National Health Service Programme (CISNS 2010),10 which proposed the substitution of the Tdap for the DTaP to the National Public Health Committee, a change that was approved by the latter. The main reason for using the Tdap vaccine in this age group is the lower reactogenicity caused by its reduced antigen content relative to the DTaP, while showing no reduction in immunogenicity for any of the three antigenic components of the vaccine (diphtheria, tetanus and pertussis).11–13
The CAV-AEP agrees with this recommendation, but it considers that the fifth dose with the Tdap vaccine must be reinforced with a sixth dose of this vaccine during adolescence, because vaccine-induced immunity to pertussis wanes over the years.3,8,14 In Spain this strategy is currently implemented in the autonomous community of Madrid and the autonomous cities of Ceuta and Melilla.2 The CAV-AEP estimates that the optimal age for this sixth dose is from 11 to 14 years. Other countries recommend giving a booster dose every 10 years (as Td, or preferably as Tdap).15 In Spain, official guidelines consider that individuals who complete their vaccination course at 14–16 years of age do not need another dose until ages 60–65, and adults are considered to be properly vaccinated if they have received 5 doses of tetanus vaccine throughout their life.16 Currently, two Tdap commercial preparations of similar characteristics are available in Spain, Boostrix® (GlaxoSmithKline) and Triaxis® (Sanofi Pasteur MSD), licensed in 2001 and 2010, respectively. Both preparations have been authorized by the AEMPS and their summaries of product characteristics indicate their use in children starting at 4 years of age.
The CAV-AEP strongly recommends immunization against pertussis with the Tdap vaccine for adults and adolescents living with newborns to provide an immune environment for the infant,17,18 universally known as the “cocooning strategy” which is being routinely practiced in some countries.19
For immunization against Hib, a single-component preparation of the vaccine is available in Spain. In this vaccine, the Hib polysaccharide capsule is conjugated to the tetanus toxoid (PRP-T), which is also the case in the combined pentavalent (DTaP-Hib-IPV) and hexavalent (DTaP-Hib-IPV-HB) preparations. The routine vaccination course recommended by the CAV-AEP has not changed from that of previous years, with administration starting at 6 weeks of life. Three doses are recommended at intervals of 4–8 weeks (2, 4 and 6 months of age). In the case of the single-component Hib vaccines in children ages 6–12 months, two doses at the time intervals given above are sufficient. A booster dose must be given at 15–18 months of age, after which subjects have been immunized with an efficacy rate nearing 100%. Two doses are recommended for unvaccinated children between 12 and 14 months of age, and one from age 15 months onward, with vaccination becoming unnecessary in immunocompetent children older than 59 months.3 Beyond this age, a single dose of the vaccine would be indicated in individuals with no vaccination history and risk factors for an invasive Hib infection: sickle cell anemia, leukemia, acquired immunodeficiencies, bone marrow transplant, and anatomical or functional asplenia.3
The inactivated poliovirus vaccine (IPV) is given as part of the hexavalent and pentavalent vaccines. The primary vaccination course in early childhood consists of 3 IPV doses at months 2, 4 and 6 of age and a fourth booster dose at 15–18 months, that must be given at least 6 months after the previous dose.3 There is also a single-component vaccine prepared with enhanced-potency inactivated poliovirus (IPVa, Salk IPV), but at the moment it is only available in Spain as an “imported medication”, and is reserved especially for unvaccinated individuals who are going to travel to polio-endemic countries.
Vaccination against group C meningococcal diseaseFor the single-component conjugate vaccines against group C meningococcal disease, the CAV-AEP recommends primary immunization with two doses in the first year of life (at 2 and 4–6 months of age) and a booster dose in the second year of life, preferably between ages 12 and 15 months. Primary vaccination in the early months of life with three doses of the meningococcal group C-CRM197 conjugate vaccine, unless a booster dose is given in the second year of life, is associated with a progressive drop in antibody levels and in bactericidal capacity a year after vaccination. Concurrently, there is a loss of vaccine efficacy and an emergence of disease cases among vaccinated children that has been documented, not only in Spain20–23 but also in other countries such as the United Kingdom.24,25 The CAV-AEP considers that many children who received that vaccination course (the one with no booster dose after 12 months of age), and who are now reaching 11 years of age, may be susceptible to group C meningococcal infections. Considering that the greatest burden of meningococcal C disease currently falls on adolescents and young adults, and the high mortality rate of this infection—37.7% thus far in Spain in year 201126—the CAV-AEP recommends an additional booster dose if epidemiological conditions justify it in the cohorts of children that have received primary vaccination but not a booster dose of meningococcal C vaccine after 12 months of age. At present, the schedule in Asturias is the only one in Spain that recommends giving a dose of meningococcal C vaccine at 14 years of age to children who have not received the previously mentioned booster dose.2
Since 2010, a new meningococcal tetravalent conjugate vaccine against serogroups A, C, W135 and Y has been available in Spain. It is for hospital use only and currently approved for administration starting at 11 years of age prior to travel to regions where the disease is endemic, such as the African meningitis belt.27
Immunization against measles, mumps and rubella (MMR)The CAV-AEP upholds the general guideline recommending the administration of two doses of the MMR vaccine after 12 months of age separated by a minimum interval of 4 weeks.1,3
In the past few years there have been measles outbreaks throughout Europe,28 Spain included, especially in young adults from areas with low vaccination coverage and children younger than 15 months that have not reached the primary vaccination age, and thus have not received any doses. Taking these epidemiological changes into account, the CAV-AEP considers that the first dose should be administered at 12 months of age, although administration any time between 12 and 15 months of age is an acceptable alternative. In Spain, eight autonomous communities and the two autonomous cities have already adopted this guideline, replacing the dose that used to be given at 15 months of age.2
The MMR vaccine is a preparation of hyper attenuated measles, mumps and rubella viruses that is highly immunogenic, achieves high rates of seroconversion (95–98%) following the administration of the first dose, and a rate of almost 100% after the second dose. Two doses are needed to achieve adequate herd immunity,29,30 since a single dose leaves 5–10% of the vaccinated children with no protection. The second dose of the MMR vaccine pursues the immunization of children that have not received the first dose of the vaccine and of children who did not produce antibodies following vaccination (primary failure). Therefore, the CAV-AEP considers that the second dose of MMR vaccine should be administered between the ages of 2 and 3 years, preferably at two. Administering this second dose at an earlier age improves compliance and also decreases the risk of susceptible children contracting the disease and of the virus spreading in the population.
A single-component vaccine against measles is not available in Spain, so all children (including the occasional vaccination of children younger than 12 months) have to be given the vaccine as MMR. The population of immigrant children who are not vaccinated against rubella and mumps should be immunized with the MMR.
The interventions to be implemented in case of an epidemiological alert due to a measles outbreak are the following28,29,31:
- -
Children younger than 6 months will be given 0.25ml/kg (40mg IgG/kg) of intramuscular nonspecific immunoglobulin in a single dose within the first 6 days post-exposure. The MMR vaccine is not indicated in infants under 6 months of age.
- -
Children ages 6–12 months will receive a dose of MMR (which will not count toward their schedule) and will be immunized again at 12–15 months, having let at least 1 month elapse, which will count as the first dose for the purposes of the vaccination schedule. If more than 72h and less than 2 weeks have elapsed since the possible exposure, children younger than 12 months will be given nonspecific immunoglobulin instead of the vaccine. Following this, 5 or 6 months later, they must be given the MMR vaccine.
- -
Individuals younger than 40 years of age with no certifiable history of the disease or of proper immunization with the MMR fitting their age are considered susceptible to the disease. It is assumed that individuals older than 40 are at very low risk, as they have acquired immunity to measles as a consequence of having it at a younger age.
- -
Unvaccinated individuals younger than 40 years who have had contact with measles cases at any point between the 4 days before and 4 days following appearance of the rash will be given a dose of MMR in the first 72h post-exposure.
- -
For children older than 3 years of age, their immunization status will be reviewed and catch-up immunizations provided as needed.
- -
Immunocompromised children exposed to measles will be given intramuscular nonspecific intramuscular immunoglobulin in doses of 0.5ml/kg (80mg IgG/kg) (maximum dose 15ml).
The CAV-AEP adheres to the recommendations of the Interterritorial Council of the Spanish National Health Service for the routine vaccination of all girls 11–14 years of age towards the prevention of cervical cancer and other precancerous lesions of the female genital tract.32 Furthermore, the CAV-AEP recommends the vaccination of all female adolescents who have not received the vaccine because they were older than the age specified by their autonomous community for routine HPV immunization.
Recently, in August 2011, changes were made in the summaries of product characteristics approved by the European Medicines Agency (EMA) for the two commercial vaccines, the quadrivalent vaccine Gardasil® (Sanofi Pasteur MSD)33 and the bivalent vaccine Cervarix® (GlaxoSmithKline).34
At present, Gardasil® is a vaccine indicated for females 9 years of age and older for the prevention of premalignant genital lesions (cervical, vulvar and vaginal) and cervical cancer causally related to certain oncogenic HPV types, and the prevention of genital warts causally related to specific HPV types.33 On the other hand, the use of Gardasil® has been authorized for males 9–26 years of age, also for the prevention of external genital warts.33 Thus, it has become the first vaccine against this virus to be authorized for use in both sexes. These indications are based on the demonstrated efficacy of Gardasil® in women from 16 to 45 years of age and in men from 16 to 26 years of age, and on the demonstrated immunogenicity of Gardasil® in male and female children and adolescents from 9 to 15 years of age. On the other hand, although the data sheet approved by the FDA contemplates the use of Gardasil® for the prevention of anal cancer caused by HPV types 16 and 18 and the prevention of anal intraepithelial neoplasia (AIN) of any grade caused by HPV types 6, 11, 16 and 18 in men and women ages 9–26 years,35,36 the EMA has yet to approve these indications, probably pending further data.
Cervarix® is a vaccine indicated for women from 10 to 25 years of age for the prevention of precancerous lesions and cancer of the cervix causally linked to certain oncogenic types of HPV.34 This clinical use is justified by the demonstrated efficacy in women from 15 to 25 years of age immunized with Cervarix® and on the immunogenicity of the vaccine in girls and women from 10 to 25 years old.
The Cervarix® data sheet includes an indication against serotypes that are not included in the vaccine, such as serotypes 31, 33 and 45, based on the data on cross-protective efficacy against cervical intraepithelial neoplasia (CIN), from the PATRICIA trial, which followed a cohort for 4 years.37 This study showed that the vaccine had an impact of 93% overall efficacy against carcinoma in situ (CIN3+), irrespective of HPV type.37 The efficacy against CIN2+ for non-vaccine oncogenic types was of 87.5% (95% CI: 68.3–96.1) for HPV31, 68.3% (95% CI: 39.7–84.4) for HPV33 and 81.9% (95% CI: 17–98.1) for HPV45.37 These data are highly relevant, since they show that the vaccine can exceed the expected overall protective efficacy against HPV-related pre-neoplastic lesions.
To achieve the maximum predicted vaccine efficacy, the HPV vaccination course requires 3 doses (Fig. 1), to be given at 0, 2 and 6 months for the quadrivalent vaccine33 and at 0, 1 and 6 months for the bivalent preparation.34 If there were any deviations from this course, administration of the vaccine should abide by the minimum intervals between doses. In the case of Gardasil®, the second dose must be administered at least 1 month after the first dose, and the third dose at least 3 months after the second one. All three doses must be administered within a year. In the case of Cervarix®, the second dose may be administered between 1 and 2.5 months after the first one, and the third dose between 5 and 12 months after the first one.
There are no data documenting the interchangeability of both vaccines against HPV, so it is recommended that the same commercial preparation be used throughout the vaccination course.33,34
Research data shows no immune interference and no significant variations in reactogenicity when these vaccines are administered at the same time as other vaccines that may be given during adolescence, such as the Tdap.38,39
Data from clinical trials40 and post-marketing surveillance41 following the distribution of over 14 million doses of the bivalent vaccine and 60 million doses of the quadrivalent vaccine, confirm the safety of these vaccines and their adequate risk-benefit ratio. In June 2009, the WHO restated the favorable safety profile of the HPV vaccine after reviewing all the available data.42 They noted that the most common adverse effects were reaction at the injection site and generalized muscle pain. Some allergic reactions were also reported in patients sensitized to one or some of the components, and there has been an increase in reports of syncope following the administration of HPV vaccines in adolescents and young adults, which are thought to be due to vasovagal reactions, which are more frequent in this age group.41,42
Since the quadrivalent vaccine has been newly authorized for male patients from 9 to 26 years of age,35 this subject must be analyzed and reviewed. The role of males in the transmission of HPV has been documented, with males showing infection rates that are higher and more prevalent across the lifespan than females, although the burden of neoplastic disease in men is much lower.43 However, the prevalence of genital warts in males is similar or slightly higher than the prevalence observed in females, and is also caused by HPV6 and 11 in over 90% of cases.43 The evidence shows a 90% efficacy in the prevention of genital warts in males33; however, there are limited data on the prevention of precancerous lesions and cancer of the anus, and in the ear, nose and throat region, although the available data show a trend that suggests protection against these conditions.33,35 Some official agencies, such as the CDC, are evaluating the recommendation of including males in vaccination programmes,36 and this assessment process must continue in the forthcoming years so that efficacy models can be complemented with the new data on the global burden of disease43 and the efficacy of the quadrivalent vaccine in males.33 Early analyses demonstrate that vaccination in males could be cost-effective in situations of low vaccination coverage in adolescent girls, although increasing the coverage of the latter would be even more cost-effective.44
Immunization against pneumococcal diseaseAs it did in previous years, the CAV-AEP maintains the recommendation for the routine vaccination against pneumococcus as the best strategy to prevent pneumococcal disease in children.
While the heptavalent pneumococcal conjugate vaccine is no longer on the market (PCV7, Prevenar®, Pfizer), two pneumococcal conjugate vaccines are now commercially available: the 10-valent PCV10 vaccine (Synflorix®, GlaxoSmithKline) and the 13-valent PCV13 vaccine (Prevenar 13®, Pfizer).
The PCV10 vaccine incorporates three additional serotypes to the seven already present in the PCV7: types 1, 5 and 7F. In this vaccine, the capsule polysaccharides of 8 of the serotypes are conjugated to protein D, a recombinant non-lipidated form of a cell-surface protein that is highly conserved in non-typeable Haemophilus influenzae, while the polysaccharides of serotypes 18C and 19F are conjugated to the tetanus and the diphtheria toxoids, respectively. It is authorized by the EMA for the prevention of invasive pneumococcal disease (IPD) and of acute otitis media (AOM) caused by S. pneumoniae in children from 6 weeks to 5 years of age.45
The PCV13 vaccine contains the 7 serotypes of the Prevenar vaccine and the following six additional serotypes: 1, 3, 5, 6A, 7F and 19A. All of them are conjugated to the CRM197 protein, a non-toxic mutant of the diphtheria toxoid. This vaccine is authorized by the EMA for the prevention of IPD, pneumonia, and AOM caused by S. pneumoniae in children from 6 weeks up to 5 years of age.46
From an epidemiological point of view, the shifts in the distribution of IPD-causing serotypes in Spain have been consolidated. At present, the serotypes contained in the PCV7 cause less than 10% of the IPD in children younger than 5 years of age in Spain.47,48 With the decline in the cases caused by serotype 5 (which is characterized by causing short outbreaks lasting a few months) in a few regions like the autonomous community of Madrid, the most prevalent serotypes causing IPD in children younger than 14 years of age are types 1, 19A and 7F, followed by others such as 3, 6A and 19F, with little variation in serotype distribution between autonomous communities.48–50 Serotypes 1 and 19A are involved in 60% of all cases of IPD in children in Madrid, but their prevalence varies as a function of age.51 Serotype 1 preferentially infects children older than 24 months of age48,52,53 and usually causes bacteraemic pneumonia and pleural empyema.54 Serotype 19A is distributed across all ages, but mostly affects children younger than 5 years of age.55 Serotype19A tends to cause different forms of IPD depending on age: in children younger than 24 months it most often causes primary bacteraemia and meningitis, while in older children it causes a significant number of bacteraemic pneumonia and pleural empyema cases.47,49,54 A recent study conducted by the Spanish Pneumococcal Reference Laboratory of the Carlos III Health Institute has demonstrated that serotypes 1, 19A and 3 cause 85% of pleural empyema cases in Spanish children.56 The increase in serotypes 1, 19A and 7F is not only happening in Spain, but also in other European countries. It has been estimated that serotype 1 accounts for 50% of IPD in children from 5 to 14 years of age in France, Belgium and Spain.55
The most striking epidemiological shift in the past few years has been the increase in serotype 19A.47,51,55,57–59 Currently, this serotype is largely associated to multi-drug resistance (resistance to 3 or more families of antibiotics), accounting for almost every case of meningitis with high-level resistance to third generation cephalosporins.48,57–59
Data are still scarce on the efficacy of the PCV10 and the PCV13 vaccines, because it has not been long since these vaccines were introduced. The monitoring of almost 3000 children vaccinated with PCV13 in a region of Alaska has shown an efficacy of 85% in decreasing the incidence of IPD caused by all serotypes 1 year after vaccination, with no record of cases caused by any of the vaccine serotypes.60 In the United Kingdom, the efficacy of the PCV13 against additional serotypes (1, 3, 5, 6A, 7F and 19A) has been greater than 50% in children of less than 2 years of age a year after starting vaccination.61 In the United States, there was also a reported decrease of over 50% in the cases of IPD caused by all serotypes, and of 70% in cases caused by the PCV13 serotypes compared to the baseline period preceding introduction of this vaccine.62 Another study from the United States showed no appreciable decrease in the morbidity caused by serotypes contained in PCV13, but not in the PCV7, except for serotype 19A, which showed a 30% decrease.63 In France, 1 year after the introduction of PVC13, the rate of nasopharyngeal carriage of serotypes 19A, 7F and 6C decreased by over 50% in vaccinated children and was not accompanied by a significant increase in the other serotypes.64
The direct prevention of IPD caused by serotypes 1, 19A, 7F and 3—not accounting for herd immunity and applying the immunological criteria of protection as defined by the World Health Organization (WHO), to pneumococcal conjugate vaccines—would result in a 50% to 60% reduction of the global burden of IPD and a marked decline in cases of empyema, bacteraemic pneumonia and occult bacteraemia, with a lower reduction in cases of meningitis and other forms of IPD. The prevention of infections by type 19A is sure to contribute to a decrease in pneumococcal antibiotic resistance.
The available epidemiologic data leads us to conclude that the PCV13 covers up to 80% of the serotypes responsible for IPD in Spanish children,47,48,51 so this is the vaccine that offers the highest serotype coverage currently in Spain.
In situations where vaccination is not universal, infants that start immunization against pneumococcus at 2 months of age should continue to follow a three-dose course of primary vaccination in the first year of life, followed by a booster dose in the second year (3+1 schedule). Primary vaccination with two doses in the absence of adequate herd immunity can leave the child at risk of infection by less immunogenic serotypes such as 6B and 23F65,66 until a booster dose is given. Therefore, in a scenario where there is no universal vaccination, the 2+1 schedule is not acceptable in individual practice for the reasons stated above.
There has been a significant change in the summary of product characteristics for the PCV10 vaccine since this Committee issued its recommendations for the 2011 immunization schedule.1 The approved age for its administration has been expanded to 5 years,45 matching the age range for the PCV13 vaccine. Children from 2 to 5 years of age with no prior history of immunization against pneumococcus can be vaccinated with the PCV10, but they must receive two doses at least 2 months apart.45
Children who have started a vaccination course with one of the two vaccines should complete the series with the same preparation. The two vaccines use different proteins for conjugation, and furthermore there are no data on interchangeability within a vaccination course.
A certain shift of IPD toward higher ages has continued to be observed in the autonomous community of Madrid52: 39% in children younger than 24 months; 37% from 24 to 59 months, and 23% in children older than 59 months.52 In this region, the PCV13 vaccine coverage for IPD cases reaches up to 87% in children from 24 to 59 months, with the more frequent serotypes being 19A (34%) and 1 (23%).52 For all of the above, the CAV-AEP recommends that children up to 59 months of age with no history of vaccination with PCV13 receive one dose of VNC13 at least 2 months apart from the last dose—if there were any—of pneumococcal vaccine, even if they have received previous doses of PCV7 or completed a VNC10 vaccination course.
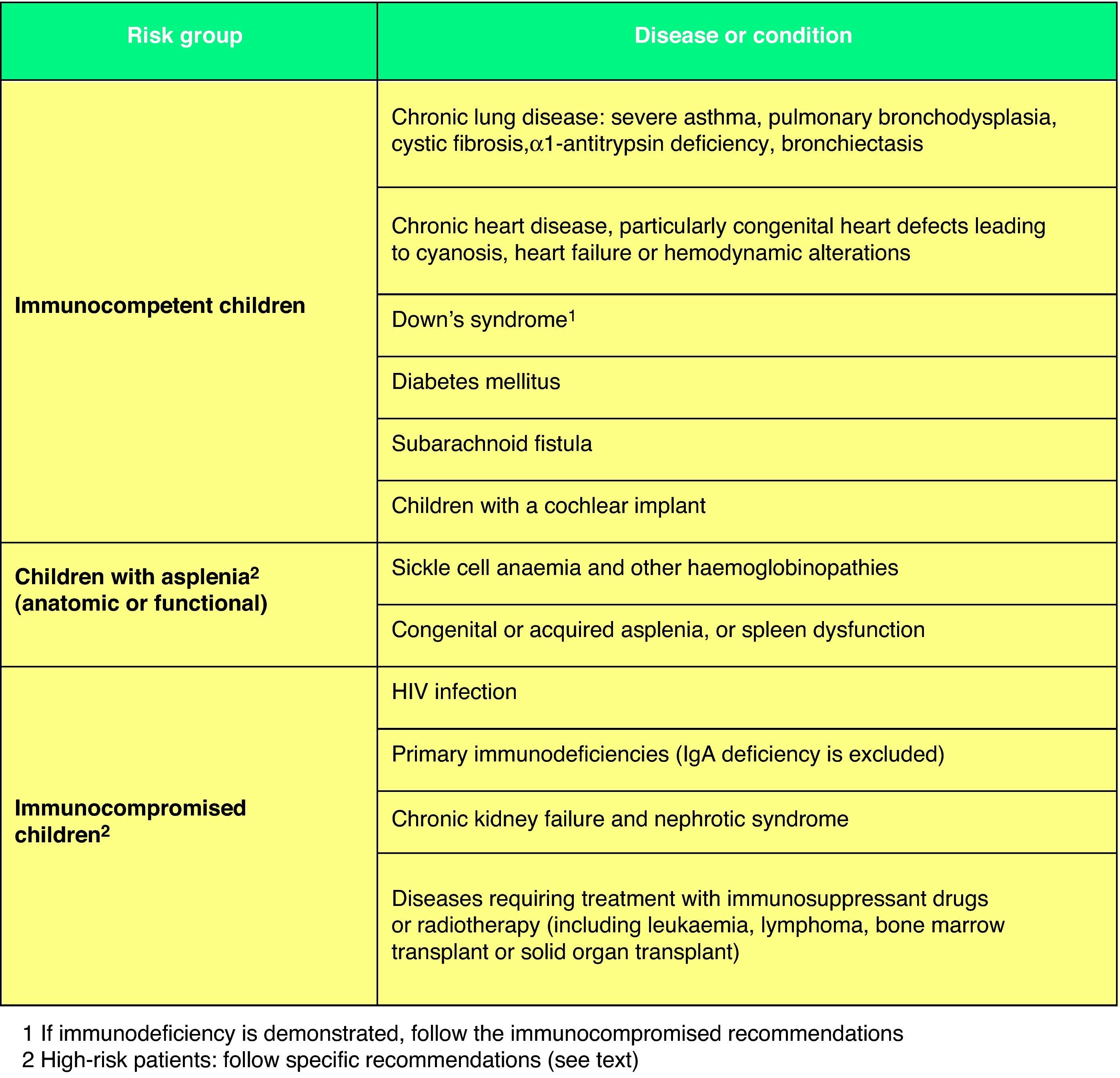
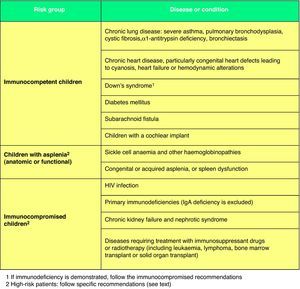
For patients at high risk of contracting an IPD (Fig. 2),67 such as immunocompromised children or children with anatomical or functional asplenia, the following recommendations have been issued: (1) a 3+1 schedule must be used in every case; (2) they must receive two doses of PCV13 in the second year of life if they have not been given at least two doses in the first year; (3) children from 2 to 5 years of age who have not received a prior dose of PCV13 must be given two doses separated by a minimum interval of 2 months. In addition, these children must complete their pneumococcal immunization with the administration of the 23-serotype pneumococcal polysaccharide vaccine (PPSV23) starting at 2 years of age, with a minimum interval of 2 months since the last administered dose of PCV13.68 They will receive the second and last dose of PPSV23 5 years later. In other children who are not immunocompromised (Fig. 2)67 but who are at high risk for contracting recurrent or severe pneumococcal disease, the guidelines for high-risk patients or the recommendations for healthy children of their autonomous community may apply, and a single dose of PPSV23 after 24 months of age is recommended, at least 2 months after of the last PCV13 dose.68
Immunization against varicellaThe CAV-AEP recommends vaccinating all children against varicella, giving the first dose between 12 and 15 months of age, preferably at 12 months, and a second dose at 2–3 years of age, if possible at 2 years. The alternative strategy of routinely vaccinating susceptible children 10–14 years of age, recommended by the Interterritorial Council of the Spanish National Health Service since 2005,32 prevents severe forms of the disease, which are more frequent among adolescents and adults, but it does not prevent the majority of varicella cases nor the majority of the complications and hospitalizations in early childhood, which are more frequent in absolute numbers.
As for the efficacy of the varicella vaccine, active monitoring in various regions of the United States where routine immunization was introduced in 1995 has demonstrated a sustained decline in cases in all age groups below 45 years of age, with the largest drop observed in children aged 0–4 years (98%).69 A decline was also seen in unvaccinated individuals, which shows that this vaccination strategy induces herd immunity.69 There was a parallel decline in hospitalizations (up to 53%), especially in children younger than 14 years,69 as well as in complications (some of which occur almost exclusively in association with varicella, as is the case of invasive Streptococcus pyogenes infections in children).70,71 A decline in mortality in the 12 years following the start of universal vaccination (1995–2007) was also recently documented in the United States, as the mortality rate for varicella fell by 88%, from 0.41/million individuals between 1990 and 1994 to 0.05/million between 2005 and 2007.72 This decline occurred in all age groups, but it was most pronounced in patients below 20 years of age.72
In the autonomous community of Madrid (Spain), where universal vaccination at 15 months of age was introduced in 2006, there was a reduction of 66% in varicella cases between 2006 and 2009, as well as of 50% in hospitalizations attributable to the virus. Routine vaccination was shown to induce herd immunity, since while the highest declines in morbidity rates occurred in the age group of 0–4 years (86%), the decrease also occurred among older children (53% in ages 5–9 years; 73% in ages 10–14 years) and young adults (56% in ages 20–24 years). So far there has been no observed age shift in varicella toward adults.73
In the autonomous community of Navarre (Spain), universal immunization against varicella was instituted in 2007 with a triple strategy: at 15 months and at 3 years, while also maintaining the vaccination of susceptible individuals at 10 years of age. In 2009, a second dose at age 3 was also added to the schedule. The incidence of varicella dropped by 93%, from 8.04 per 1000 inhabitants in 2006 to 0.56 per 1000 inhabitants in 2010 (P<.0001). In children aged 1–6 years (vaccinated cohorts), the incidence of varicella declined by 96.3%. In cohorts of children vaccinated at 10 and 14 years of age, there was also an observed decline of 93.6% in children 10–14 years of age, and of 85.0% in children 15–19 years of age. In the unvaccinated age groups there are observed declines of 88.2% in children younger than a year, of 73.3% in children ages 7–9 years, and of 84.6% in individuals older than 20 years of age. In 2006 there were 25 hospital admissions for varicella in Navarre, and in 2009 this figure had dropped to 7. The hospitalization rate decreased by 73%. In conclusion, the introduction in Navarre of universal vaccination against varicella has led to a rapid and very pronounced decline in the incidence of varicella, both in vaccinated and unvaccinated individuals.74
In the United States, there were outbreaks of varicella or a relatively high number of cases of the disease over the years after the implementation of a single-dose vaccination schedule, even in areas with vaccination coverage rates as high as 90%.75–77 Therefore, it was estimated that 10 years after vaccination the efficacy of a single-dose schedule can be as low as 72% and that one in every 5 vaccinated children will be susceptible to the disease.77–79 It was also observed that the varicella cases in children vaccinated with a single dose tended to involve mild forms of the disease, with few or no systemic symptoms, confirming that the efficacy of the vaccine against severe forms of the disease is higher than 95%. However, with increasing time after vaccination, it is observed that not only is there an increase in the number of cases in vaccinated children, but that severe cases that may even require hospitalization also become more frequent. Varicella cases in vaccinated children may be due both to the waning of vaccine-induced immunity and to primary vaccine failure, which in some studies occurred in up to 24% of vaccinated subjects.77,80
For these reasons, the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Pediatrics have recommended a vaccination programme with two doses of varicella vaccine since 2006. In this schedule the first dose is given between 12 and 15 months of age and the second at 4–6 years of age.81,82 Giving the second dose of the vaccine achieves seroprotection rates over 95% and an antibody response that can be up to 15 times higher than the one obtained with the initial dose.83 With this approach, the obtained immune response comes much closer to the response to natural infection than the response obtained with a single dose, there is a sharp drop in cases of varicella caused by waning immunity in vaccinated children, and primary vaccine failures that may have occurred with a single dose are remediated.81,83
Some mathematical models estimate that a single dose of the vaccine with a 90% vaccination coverage rate would achieve a decline in varicella cases of approximately 65% in the years following introduction of the vaccine, and that the addition of a second dose would increase the efficacy of the vaccine by 22%.84 On this subject, we must stress the importance of achieving vaccination rates higher than 90% for the two doses in the first years of the programme, since otherwise there is a risk that a pocket of susceptible subjects will emerge and considerably increase the burden of disease in adulthood. Without a doubt, the best way to achieve these coverage rates would be for the vaccination programme to be funded by the public health services, as happens in the autonomous communities of Madrid and Navarre, and the autonomous cities of Ceuta and Melilla.
In the context of the universal immunization against varicella included in the routine schedule for the second year of life, the vaccination of all children, regardless of whether they have or have not had the disease in the first year of life, should be taken into consideration for strategic reasons.
When it comes to the cost-efficiency of the two-dose strategy, the United States studies support the use of routine vaccination.82 However, given that cost-effectiveness studies cannot be extrapolated fully from one country to another, it would be desirable to undertake analyses of this parameter in our environment, to confirm the positive impact of this strategy with regards healthcare costs, as we did for the single-dose schedule.
The potential increase of the incidence of herpes zoster among individuals that had varicella during childhood is certainly an issue to consider, since theoretically the high vaccination coverage rates would bring the circulation of the wild virus to a minimum or eliminate it altogether, and the exogenous effort exerted to maintain the latency of the virus in individuals who had the disease as children may be eliminated along with it. This fact, which at the moment remains hypothetical, could lead to a significant increase in herpes zoster cases that would persist until the adult population became predominantly composed of vaccine-induced immune individuals not hosting the wild virus. At any rate, some publications that have documented a slight increase in herpes zoster cases associated to the varicella immunization programme are already proposing vaccinating against herpes zoster starting at 50 years of age.85–88
Analyzing all these facts and in light of the epidemiological data of the Spanish autonomous communities and the countries that have introduced universal vaccination against varicella, the CAV-AEP considers that the two-dose routine vaccination strategy, with a first dose at 12–15 months of age, preferably at 12 months, and a second dose at 2–3 years of age, preferably at 2, is the most appropriate. It is recommended that both doses be co-administered with the MMR vaccine (see the previous section on MMR). We must remember that since both are live vaccines, it is advisable to give them on the same day and at different anatomical sites, but if this were not possible, they should be given at least 1 month apart. Giving that the second dose is most effective, not only to avoid a greater number of cases and their complications in children, but also to ensure vaccination coverage rates above 90% that can prevent varicella cases in adolescents and adults.
Selective immunization during infancy of children who are at risk of severe varicella and of the healthy individuals in their immediate environment, as well as the universal vaccination of susceptible adolescents later in life, is traditionally associated to low coverage rates and an age shift of the disease to adulthood.
However, it is interesting to note that for children at risk of severe varicella and the healthy individuals around them, the two doses of the vaccine must be given within a shorter interval than the one proposed for the general schedule so they can gain protection quickly and to avoid potential primary vaccine failures. In this regard, the current recommendation is that children less than 13 years of age belonging to this group, who could be given the second dose a month after the first one, actually get it at least 3 months later, while children older than 13 years should receive the second dose 1 month after the first one. The Committee will assess whether to generalize the shortening of the interval between doses in the future, a measure that has already been implemented in the autonomous cities of Ceuta and Melilla.
Currently two vaccines against varicella are available in Spain: Varivax® (Sanofi Pasteur MSD) and Varilrix® (GlaxoSmithkline). Since September 2009, the latter has been authorized by the Spanish Agency for Medicine and Health Products (AEMPS) only for hospital use, and thus it is not currently available for commercial sale outside hospitals, with its administration being restricted to hospital pharmacy services. This difference in marketing authorization was not based on any variations in the efficacy, immunogenicity or safety between the two vaccines. Therefore, although ideally the two doses given in or outside of the hospital to comply with the schedule would be of the same commercial preparation for any given patient, if a child had been given the first dose as Varilrix® in the extra-hospital environment, and due to the aforementioned circumstances, he/she could not be given a second dose of the same preparation, it is advisable that the course be completed (second dose) with Varivax®.
Immunization against rotavirusRotavirus has been identified as the primary causal agent of acute gastroenteritis (AGE) in children worldwide, particularly in children younger than 5 years of age. It is associated with high morbidity rates in all countries and high mortality rates in low-income countries.89,90 In industrialized countries, infection by rotavirus poses a high healthcare burden, with a high number of hospital admissions and medical visits.
The best preventative strategy against the disease is universal vaccination.91–93 The top priority is protection against the severe forms of AGE to lower the burden of disease and the use of resources. Countries that have implemented vaccination have shown a documented decline in the activity of circulating rotavirus and a reduction in the expected number of rotavirus-related hospital admissions,94–97 and even a decrease in the mortality rate of AGE of any etiology in children from 0 to 59 months of age.98
There are two rotavirus vaccines for which clinical trials have demonstrated their efficacy, immunogenicity, safety, and low reactogenicity. They are RotaTeq® (Sanofi Pasteur MSD), and Rotarix® (GlaxoSmithKline).
RotaTeq® is a pentavalent vaccine containing five human-bovine reassortant rotavirus strains. The immunization course consists of three oral doses starting at 6–12 weeks of age, with the subsequent doses at intervals of at least 4 weeks. The maximum age recommended for the first dose is 12 weeks, and 26 weeks (6 months) for the last dose in Europe.
Rotarix® is a monovalent live attenuated vaccine obtained from a human virus strain. It is administered orally starting at 6–12 weeks of age in two doses separated by a minimum of four weeks. The vaccination course must start at 12 weeks of age at the latest, and must be completed before 24 weeks of age (6 months).
In developed countries that have introduced routine immunization against rotavirus, there has been evidence of a significant reduction in the number of hospitalizations due to this virus in children younger than 5 years of age.99,100 In the United States there has been a documented 2–4-month delay in the onset of rotavirus-related AGE in the seasons of the 2007–2010 period compared to the 1991–2006 period, no activity peak was noted for the 2010 season, and there was a decrease of over 50% in rotavirus activity, as well as a decrease of over 80% in the rotavirus detection rate.95 Another recent study from the United States shows a 25–33% reduction in hospitalizations for AGE after introduction of the vaccine. Reductions in AGE-related hospitalizations due to rotavirus have ranged from 60% to 75%.101
Studies recently published in Spain also confirm a significant reduction in hospitalizations attributable to rotavirus infection since the marketing of the vaccine in 2006, showing a direct relationship between vaccine coverage rates and declines in hospital admissions.102
In Austria (the first European country to include the rotavirus vaccine in its routine immunization schedule), there has been a 96.6% reduction in rotavirus cases since the introduction of the programme, with an estimated coverage rate of 74%.103 This study highlights a drop in the number of AGE cases due to rotavirus in infants younger than 3 months who had not yet been vaccinated or had received just a single dose of the vaccine, suggesting that the programme has resulted in a degree of herd immunity.103
In Europe, both the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), and the European Society for Paediatric Infectious Diseases (ESPID) have recommended the universal vaccination against rotavirus of all healthy European children since 2008.104 This vaccine has also been part of the routine immunization schedule recommended by CDC of the United States since 2006.92 In Spain, rotavirus vaccination is not included in the schedule proposed by the Interterritorial Council of the Spanish National Health Service.32 However, the CAV-AEP has included it in its recommendations since 2008.
South American countries have reported a 60–80% reduction in rotavirus-specific AGE-related hospitalizations, and a 30–40% reduction in the mortality rate due to all-cause diarrhea in children younger than 5 years.105 A case-control study done in Mexico and Brazil estimated that immunization against rotavirus has prevented about 80,000 hospitalizations and 1300 deaths caused by diarrhea each year. There was a reported increase in intussusception cases of one in every 51,000 vaccinated children and 1 per 80,000 vaccine doses respectively.106
At first, the WHO only recommended including the rotavirus vaccine in national immunization programmes for countries where efficacy data suggested there would be a significant positive public health impact, but once efficacy data from Africa and Asia became available, the recommendation was expanded to every country in the world.93 An updated review of rotavirus immunization data showed a vaccine efficacy rate comparable to the one found in pre-marketing clinical trials, with rates ranging from 80 to 98% in industrialized countries and from 39 to 77% in African and Asian countries.107
A health alert emerged in 2010 when porcine circovirus DNA was found in rotavirus vaccines (Rotarix® y RotaTeq®).108 The WHO and the pharmaceutical regulatory agencies of Europe (EMA) and the United States (the Food and Drug Administration, FDA) started an exhaustive research process, launching several studies to assess the implications of the presence of porcine circovirus particles in these vaccines. The unanimous conclusion was that the particles did not pose a threat to human health, thus establishing that there was no reason to restrict the use of these vaccines.109–112 Porcine circoviruses do not infect or cause disease in humans, and they are commonly found in meat products and in the trypsin used in vaccine development. In fact, the possibility that the presence of circovirus in the Rotarix® vaccine could infect human cells has been ruled out recently.113
In Spain, several scientific associations, such as the AEP, the Spanish Association of Vaccinology (AEV), the Spanish Society of Paediatric Infectious Diseases (SEIP) and the Spanish Society for Paediatric Gastroenterology, Hepatology and Nutrition (SEGHNP), issued a consensus document in July 2010, and a second, updated document in December 2010, stating that all the available data confirm that the presence of porcine circovirus in these vaccines does not pose a threat to the health of the children that have received them and does not affect the safety or the efficacy of the preparations, and that vaccination against rotavirus remains an advisable preventative strategy for every child in Spain.114
The extensive pre-marketing clinical trials of both rotavirus vaccines, which involved over 120,000 children, showed no correlation between intussusception and either vaccine, nor any other clinically relevant adverse effects.115,116 Some studies published in 2001 that use post-marketing surveillance data for both vaccines showed that there were a few more cases of intussusception than expected within the first week post-vaccination compared to baseline incidence rates, although no difference was found between vaccinated and unvaccinated children. Data from the national vaccination programme in Australia showed some evidence of an elevated risk for intussusception following the first dose of both vaccines, although no overall increase in the incidence of intussusception was found in the population of vaccinated children.117 Also, evidence from a post-marketing safety study conducted in Mexico seems to support that the risk for intussusception increases by a factor of 2–6 in the 30 days following the initial dose, with cases being most frequent in days 1–7 following its administration.106
These data warrant the sustained post-marketing surveillance of the rotavirus vaccines. However, the benefits obtained from immunization against rotavirus in the form of declining morbidity and mortality rates continue to greatly offset the hypothetical risks we have discussed, an opinion endorsed in December 2010 by the WHO, which extended its recommendation for the universal immunization against rotavirus.118,119
Two rotavirus vaccines have been licensed in Spain since 2006. Both vaccines continue to be authorized for use in Spain with the same therapeutic indications and clinical uses, although only RotaTeq® is currently available in the pharmaceutical distribution channels following the November 2010 ruling of the Spanish Agency for Medicine and Health Products (AEMPS).120
Given the high morbidity rates and elevated healthcare associated with this disease, this Committee continues to regard the vaccination of all infants against rotavirus as an unquestionable health benefit.
Immunization against seasonal influenzaThe CAV-AEP considers that the influenza vaccine is a particularly beneficial strategy when it targets children and adults in at-risk population subsets. These subsets consists of individuals with a pre-existing condition or undergoing medical treatment who may develop more severe and complex forms of influenza or experience the destabilization of their underlying condition upon infection with the virus, which would increase their risk of dying. The expanded facts about this immunization can be consulted in the annual report prepared by this Committee prior to the beginning of the influenza season.121
Each year, the WHO determines which influenza strains will be included in the seasonal vaccines. An influenza vaccine with the same composition as the vaccine of the previous 2010–2011 campaign will be used in the Northern Hemisphere for the 2011–2012 season.122 This coincidence does not imply a change in the recommendation for annual immunization, and therefore vaccination is still recommended for individuals vaccinated in the 2010–2011 season.
For children and adolescents, the CAV-AEP recommends the influenza vaccination for:
- 1)
Risk groups: children over 6 months of age and adolescents with the following conditions or underlying diseases:
- -
Chronic respiratory disease (e.g. cystic fibrosis, bronchopulmonary dysplasia, asthma and bronchial hyperreactivity, etc.).
- -
Severe cardiovascular disease (congenital or acquired).
- -
Chronic metabolic disease (e.g. diabetes, congenital metabolic defects, etc.).
- -
Chronic kidney (e.g. renal failure, nephrotic syndrome, etc.) or liver disease.
- -
Chronic inflammatory bowel disease.
- -
Congenital or acquired immunodeficiency.
- -
Functional or anatomical asplenia.
- -
Cancer.
- -
Moderate or severe hematological disease (e.g. haemoglobinopathy, leukemia, etc.).
- -
Chronic neuromuscular disease and moderate to severe encephalopathy.
- -
Moderate or severe malnutrition.
- -
Morbid obesity (BMI greater than or equal to three standard deviations above the mean).
- -
Down's syndrome and other severe chromosomal disorders.
- -
Ongoing treatment with acetylsalicylic acid (due to risk of Reye syndrome by wild influenza infection).
- -
Pregnancy in adolescents.
- 2)
Healthy children over 6 months of age and healthy adolescents who live with patients at risk.
- -
Vaccination is recommended for healthy children over 6 months of age and healthy adolescents who have no underlying disease but have household contact with (i.e. live with) patients (children or adults) belonging to risk groups.
- 3)
Adults in contact with children and adolescents belonging to risk groups.
- -
Seasonal influenza vaccination is highly advisable for all adults who have household contact with (i.e. live with or care for) children and adolescents who belong to risk groups (see Section 1 above). The recommendation for influenza vaccination is particularly emphasized for medical personnel working with children.
The CAV-AEP believes that vaccination against influenza in all of these patients and their household contacts offers clear and unquestionable health benefits.
Since children are the spreaders of the influenza virus in the community,123 shed larger amounts of virus and for longer periods than adults,124 the highest incidence rates of influenza occur in children under 15 years of age125 and the average hospitalization rate for children under 5 years of age is around 1 per 1000 healthy children,126 the CAV-AEP considers that children older than 6 months who do not belong to the above risk groups can be vaccinated against the seasonal influenza at the request of their parents or the recommendation of their pediatrician. This preventative approach has unquestionable health benefits, since it can offer direct individual protection to the child or adolescent, and indirectly promote protection of the household and the community.
At present, the implementation of a universal childhood influenza vaccination programme in Spain using the available vaccines faces challenges due to a number of complications and shortcomings: (1) the need to add an annual intramuscular vaccine to the immunization schedule, with the problems inherent in implementation and compliance, (2) the limited efficacy of the trivalent inactivated influenza vaccine in children under 2 years of age, which may be improved eventually,127 and (3) its high cost and the insufficient data on its efficacy in children.
In children under 9 years of age who are being vaccinated for the first time, two doses of the vaccine at least 4 weeks apart are needed to obtain optimal protection against the flu. The first dose should be administered as soon as the vaccine becomes available to ensure that both doses are received before the beginning of influenza activity, since protection is highest when both doses are administered in the same influenza season. If there is a history of correct vaccination with two doses in a previous season, a single dose in the current season will suffice. Likewise, if they received a single dose of the influenza vaccine for the first time in the last season (2010–2011), they should only receive one dose of influenza vaccine in the current season (2011–2012)128 since the composition of the vaccine is identical for both campaigns.122 In children 9 years of age or older, if indicated, a single dose of the vaccine per season will suffice.128
The only currently available vaccines approved for use in children less than 18 years of age in Spain are the inactivated trivalent preparations,129 prepared by inoculation of cultures in embryonated chicken eggs to be administered intramuscularly.
New influenza vaccinesNumerous commercial preparations of the influenza vaccine will be available for the forthcoming 2011–2012 season, all with the same antigenic composition. Various innovative preparations (live attenuated vaccines, adjuvant vaccines, tetravalent vaccines, and cell culture vaccines) with alternative routes of administration (intradermal, intranasal, etc.) are being gradually incorporated. It is expected that the future availability of these preparations in Spain will open new horizons in the immunization of children against influenza.
Immunization against hepatitis AThe CAV-AEP recommends vaccination against hepatitis A for pre-exposure prophylaxis in children older than 12 months of age at high risk for infection:
- •
Travelling to countries with medium to high endemicity of hepatitis A, especially if they are immigrant children visiting their countries of origin.
- •
Residents of closed institutions and their careers.
- •
Children with Down's syndrome and their caregivers.
- •
Recurrent recipients of haemoderivatives.
- •
Particularly indicated in children and adolescents at increased risk for acute liver failure following infection with hepatitis A, such as:
- ∘
Patients awaiting a liver transplant or with chronic liver disease.
- ∘
Patients seropositive for hepatitis B or C, or undergoing sustained treatment with hepatotoxic drugs.
- ∘
Indications for post-exposure prophylaxis in the 14 days following exposure include the following:
- •
Household contact with an acute hepatitis A infection case.
- •
Preferentially in the event of outbreaks in child care centres.
The vaccination schedule for both pre- and post-exposure prophylaxis consists of two doses, starting at 12 months, separated by an interval of at least 6–12 months.130 For travelers it is recommended that the first dose be administered at least 1 month prior to travelling to the endemic region.
In most autonomous communities in Spain, vaccination against hepatitis A is recommended only for individuals in at-risk groups, except in Ceuta and Melilla, which incorporated the universal vaccination against hepatitis A into the immunization schedule in 2000. Previously, in 1998, Catalonia recommended the universal vaccination of 12-year-old pre-adolescents against hepatitis A, to be implemented in schools with the administration of a combined HA-HB vaccine. The effectiveness of this measure against hepatitis A has been quite significant, with a 97% drop in the incidence of cases in vaccinated cohorts and considerable declines in unvaccinated children, probably as a result of herd immunity. As a result, it was decided that the programme would continue until the 2013–2014 academic year, when the cohorts of children vaccinated against hepatitis B in the first year of life will reach the school year in which the combined HA-HB preparation is administered.131–134
As happened with the strategy of selective vaccination against hepatitis B, vaccinating at-risk individuals against hepatitis A will have little impact on the incidence of the disease, since it can only prevent a small percentage of total cases. Only universal vaccination has the potential of significantly reducing the incidence of the disease. Furthermore, since there are no non-human reservoirs for hepatitis A and this virus does not cause chronic infections, universal vaccination has the potential of eradicating hepatitis A disease in a region or an entire country.
In short, the CAV-AEP maintains the recommendation of vaccinating individuals at risk for hepatitis A, and considers that universal childhood vaccination against hepatitis A could be the best strategy for the eventual eradication of this infectious disease.
Forthcoming immunizations: meningococcal B vaccinesFor years, numerous research projects have been devoted to the development of an efficient vaccine against serogroup B meningococcal disease. The early vaccines that were developed elicited a poor immune response and showed low efficacy rates, particularly in children below 4 years of age, as well as little cross-protection against heterologous strains, so they have been used sparingly.
The latest lines of investigation are based on recombinant technology and reverse vaccinology.135 These new technologies have given rise to two vaccines currently in different phases of development:
- -
Multicomponent meningococcal serogroup B vaccine (rMenB+OMV or 4CMenB), developed by Novartis. This vaccine has completed pre-marketing clinical trials and was submitted to the EMA for its marketing authorization in December 2010. It contains several antigens associated to pathogenicity (fHBP, NadA and NHBA), combined with outer membrane vesicles (OMV) from a vaccine previously developed in New Zealand (strain NZ98/254) that serves the function of an immunomodulator. The data presented were based on various clinical trials that enrolled over 8000 infants, young children, adolescents and adults. The primary vaccination course used in the trials consisted of 3 doses (at 2, 4, 6 months). The results of the trials show that the vaccine induces a good immune response in infants when administered alone or in combination with other scheduled vaccines, and that it has an acceptable tolerability profile. The vaccine is equally immunogenic when it is given as a booster in the second year of life to children previously vaccinated, or when two doses are administered 2 months apart between 12 and 15 months of age in previously unvaccinated children. It also elicits a powerful immune response in adolescents and adults. Recent data indicate that this vaccine can provide protection against 75–80% of the meningococcal B strains that cause invasive disease in Europe.136
- -
The other vaccine under development, from the Pfizer Laboratories, is a bivalent vaccine composed of two variants (A05 and B01) of the outer membrane lipoprotein family known as LP2086. Clinical trials in adolescents and adults show a good immune response following administration of two doses. There are still no data from the clinical trials of the vaccine in the paediatric population.137
In summary, it seems that the introduction and commercialization of vaccines effective against serogroup B meningococcal disease are on their way. These preparations fit the profile for routine vaccinations, but we still need to confirm that they cover the strains that circulate in our environment.
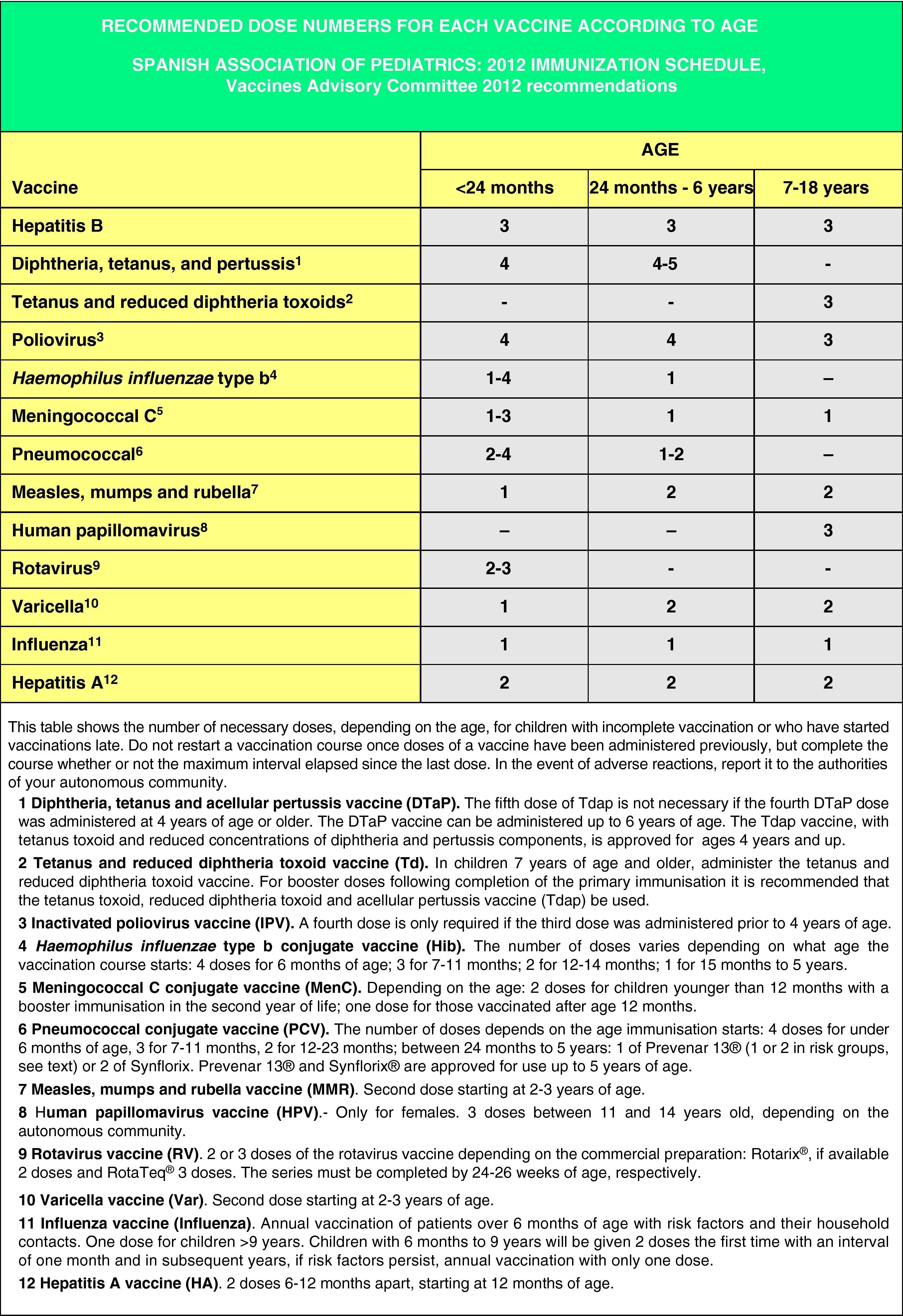
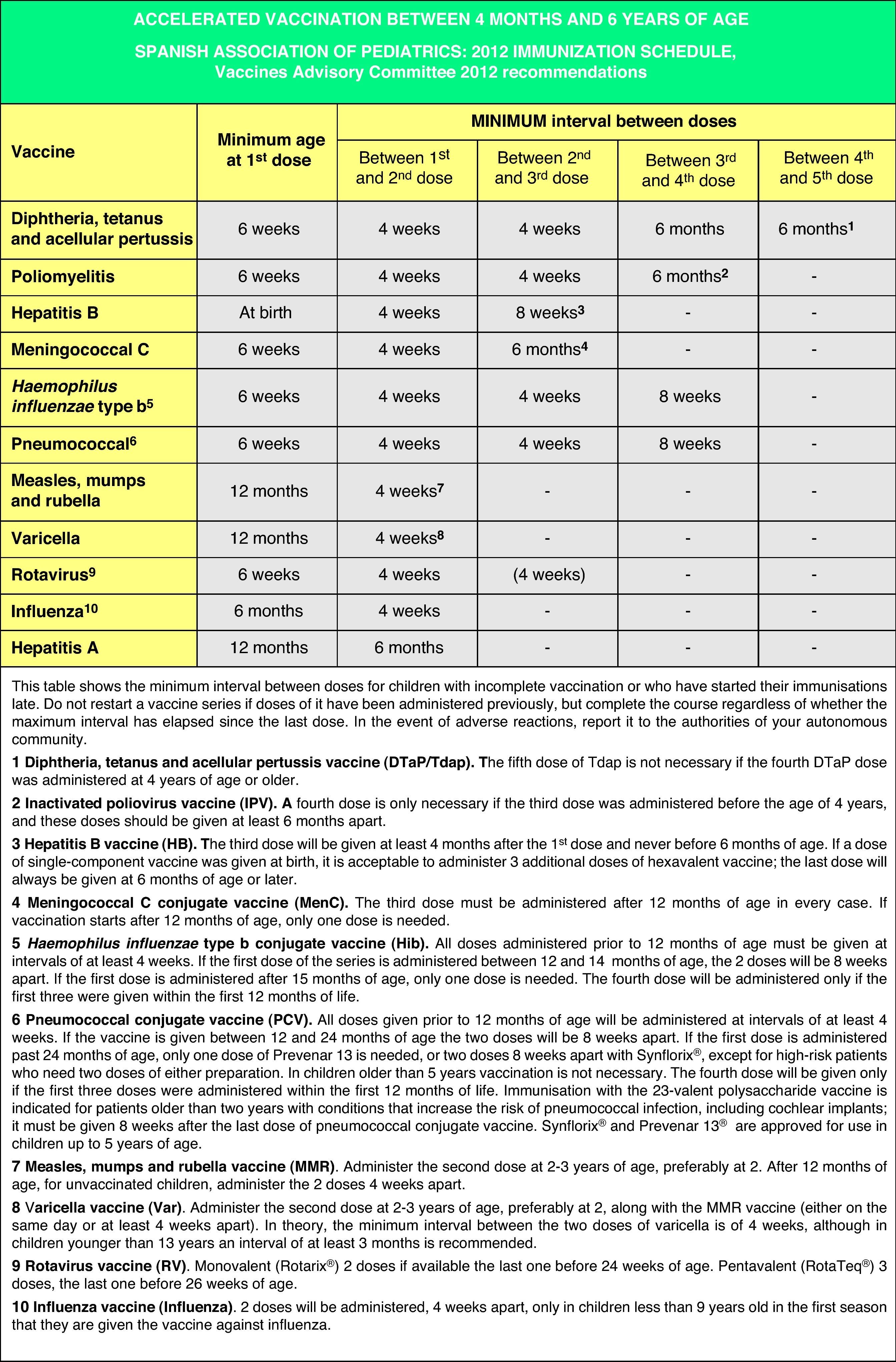
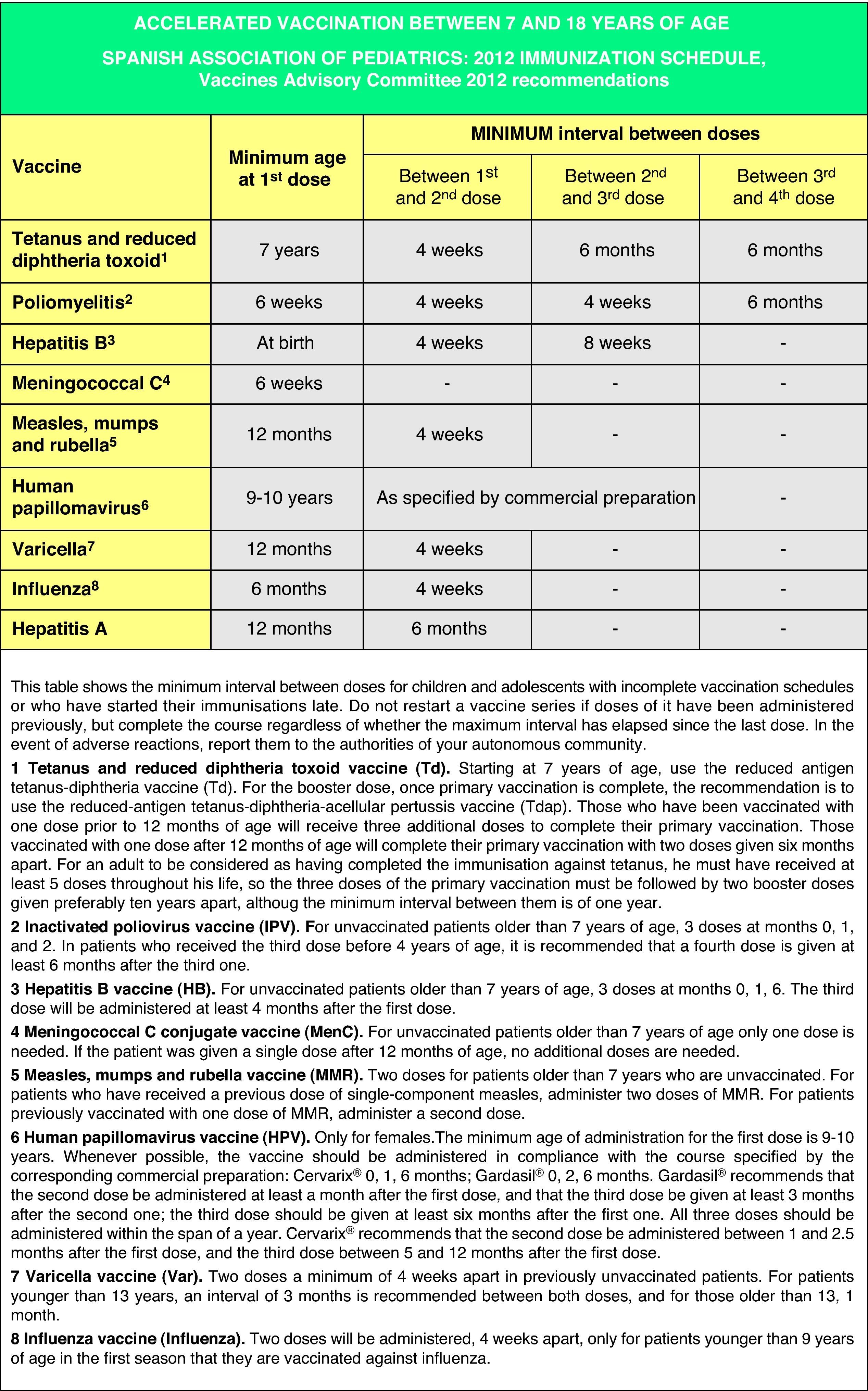
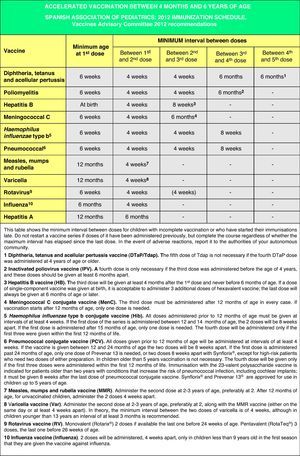
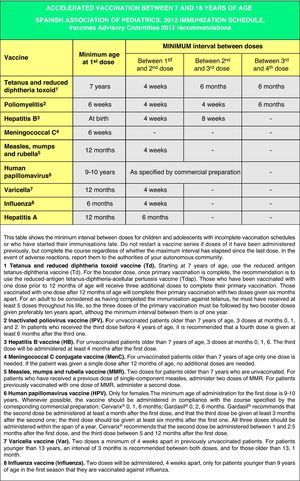
Accelerated immunization schedules for children and adolescents with incomplete vaccinationIn many instances it is necessary to vaccinate children who have not received any prior vaccines or who have not followed an immunization schedule regularly, have started immunizations late, have stopped their vaccinations, or have been vaccinated in their countries of origin following a programme that diverges from the current schedule. In all of these children, vaccinations must be adjusted to comply with the local immunization schedule. This committee has prepared a series of tables to guide the implementation of accelerated vaccination schemes in children and adolescents with incomplete vaccination (Figs. 3–5).
These accelerated schedules have been designed as tools to aid pediatricians in their daily practice. They are based on the recommendations of various scientific societies and experts, and the following guidelines must be taken into account for their interpretation:138,139
- •
The age of the child and the corresponding number of doses required for correct vaccination (Fig. 3). Previously administered doses, if any, should count toward the vaccination course, as long as they match the minimum age and intervals of administration. A vaccination course will not be restarted if the child has received any previous valid doses. The number of doses needed to bring the schedule up to date will be calculated by subtracting the number of doses already received from the number of doses advised for that age group in the official schedule.
- •
All doses properly recorded or identified will be considered valid. In cases where there is no documentation of the administered vaccines and where questioning the patient cannot faithfully establish which vaccines were given, there is the option of administering all the vaccines that are appropriate for an unvaccinated individual in that age group.
- •
The minimum interval between doses must be upheld to achieve an adequate immune response and to consider the vaccination valid. Applying these intervals facilitates the completion of the immunization schedule (accelerated course) in a time-efficient manner to reach an adequate immunization status as fast as possible. From this point on, it is preferable that, instead of using minimum intervals, vaccination proceeds according to the intervals specified in the routine schedule.
- •
As many vaccines as possible will be administered simultaneously in different anatomical sites. Combined vaccines will be used preferentially (to reduce the number of injections). If for any reason all the vaccines could not be given at the same time (reluctance of child, parents or tutors, elevated number of pending doses, or unavailability of any of the commercial preparations) and the child had a permanent address and was expected to return to the practice, the vaccines to be administered first will be those against the pathology that poses the highest risk given the child's age group and the epidemiology of his environment, and those against diseases for which the child has yet to receive his first dose.
Figs. 4 and 5 show the minimum intervals and the number of doses needed for the vaccinations recommended by the CAV-AEP in children between 4 months and 6 years of age, and children and adolescents between 7 and 18 years of age, respectively. The footnotes of these tables must be read to interpret the figures correctly, since the information contained in these footnotes clarifies some aspects that apply to specific situations.
Conflict of interestsDMP has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, as a researcher for clinical trials for GlaxoSmithKline and as a consultant on an Astra-Zeneca Advisory Board.
FJAG has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD.
JAF has collaborated in educational activities and as a researcher in clinical trials funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD.
FBC has collaborated in educational activities funded by GlaxoSmithKline and Sanofi Pasteur MSD and as a researcher in clinical trials for GlaxoSmithKline and Baxter.
MJCO has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD and as a researcher in clinical trials for Pfizer.
JMCR has collaborated in educational activities funded by GlaxoSmithKline, Sanofi Pasteur MSD and Novartis.
JGH has collaborated in educational activities funded by GlaxoSmithKline, Pfizer, and Sanofi Pasteur MSD.
THSM has collaborated in educational activities funded by Pfizer and Sanofi Pasteur MSD.
MMM has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD and as a researcher in clinical trials for GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD.
LOC has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD and as a researcher in clinical trials for GlaxoSmithKline.
JRC has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD and as a researcher in clinical trials for GlaxoSmithKline and Pfizer.
Appendix 1. Members of the Spanish Association of Pediatrics Vaccines Advisory CommitteeDavid Moreno-Pérez (DMP), Francisco José Álvarez García (FJAG), Javier Arístegui Fernández (JAF), Francisco Barrio Corrales (FBC), M. José Cilleruelo Ortega (MJCO), José María Corretger Rauet (JMCR), José González-Hachero (JGH), Teresa Hernández-Sampelayo Matos (THSM), Manuel Merino Moína (MMM), Luis Ortigosa del Castillo (LOC), Jesús Ruiz-Contreras (JRC).