Type 1 diabetes (T1DM) is an autoimmune disease whose late diagnosis can lead to serious complications such as diabetic ketoacidosis, especially in children. The presence of specific autoantibodies allows for the identification of a presymptomatic phase, opening the door to screening strategies targeting populations at high genetic risk, such as first-degree relatives. This document presents the consensus recommendations of the Spanish Diabetes Society (SED), the Spanish Society of Endocrinology and Nutrition (SEEN) and the Spanish Society of Paediatric Endocrinology (SEEP) on the screening, staging and monitoring of T1DM in preclinical stages. Early identification of the disease will enable a personalised approach to be established, promote health education and, eventually, consider therapeutic interventions that may delay progression to the symptomatic phase. This consensus seeks to establish a common framework for clinical action based on the available evidence, with clear recommendations for its proper implementation.
La diabetes tipo 1 (DT1) es una enfermedad autoinmune cuyo diagnóstico tardío puede conllevar complicaciones graves como la cetoacidosis diabética, especialmente en niños. La presencia de autoanticuerpos específicos permite identificar una fase presintomática, abriendo la puerta a estrategias de cribado dirigidas a poblaciones de riesgo genético elevado, como los familiares de primer grado. Este documento presenta las recomendaciones consensuadas de la Sociedad Española de Diabetes (SED), la Sociedad Española de Endocrinología y Nutrición (SEEN) y la Sociedad Española de Endocrinología Pediátrica (SEEP) sobre el cribado, la estadificación y el seguimiento de la DT1 en fases preclínicas. La identificación temprana de la enfermedad permitirá establecer un abordaje personalizado, promover la educación en salud y, eventualmente, considerar intervenciones terapéuticas que puedan retrasar la progresión hacia la fase sintomática. Este consenso busca establecer un marco común de actuación clínica basado en la evidencia disponible, con recomendaciones claras para su adecuada implementación.
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease characterized by the progressive destruction of pancreatic beta cells, ultimately resulting in a virtually absolute insulin deficiency.1
Early detection of this autoimmune process has become a priority,2,3 not only because it facilitates the earlier diagnosis of T1DM, but also because it enables the implementation of preventive strategies aimed at reducing acute complications at diagnosis and preserving residual beta-cell function.4,5
To facilitate and standardize the management of the presymptomatic phases of the disease, this document presents the consensus position of the Spanish Diabetes Society (SED), the Spanish Society of Endocrinology and Nutrition (SEEN), and the Spanish Society of Paediatric Endocrinology (SEEP) on the screening, staging, and monitoring of T1DM in preclinical stages. The full document is available in the Appendix (Supplementary data).
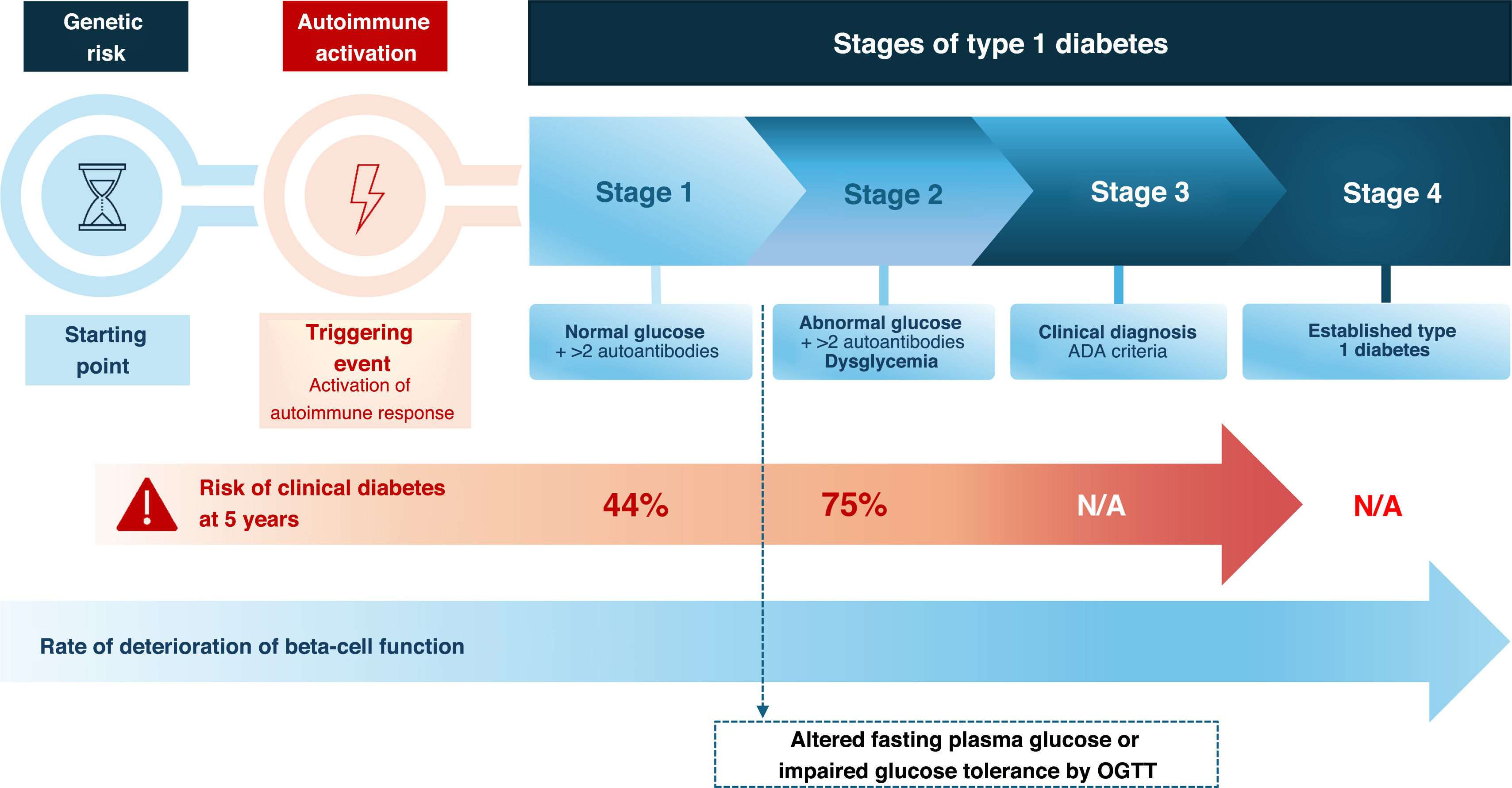
Stages of T1DM and risk of progressionT1DM progresses through several stages, from autoimmune activation in genetically predisposed individuals to the onset of clinical symptoms.6 The current staging of T1DM is shown in Fig. 1. Once 2 or more autoantibodies against beta-cell antigens are detected (stage 1), the lifetime probability of progression to clinical diabetes is high, although at this stage glucose levels remain normal and individuals are asymptomatic. Stage 1 is usually followed by the development of impaired fasting glucose or glucose intolerance (dysglycemia), which defines stage 2. However, this stage may not be detected if progression to stage 3 occurs rapidly.
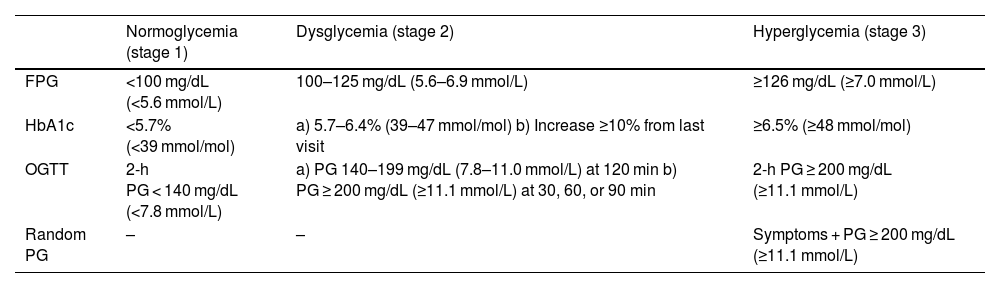
The American Diabetes Association (ADA) criteria7 for defining normoglycemia, dysglycemia, and hyperglycemia are based on fasting plasma glucose (FPG), 2-h plasma glucose during a 75-g oral glucose tolerance test (OGTT), and HbA1c (Table 1). Alternatively, to diagnose stage 2 (dysglycemia), intermediate time points during the OGTT may be used if plasma glucose values are ≥200 mg/dL (≥11.1 mmol/L) at 30, 60, or 90 min. Random plasma glucose can also be measured to diagnose hyperglycemia in symptomatic individuals (Table 1).
ADA Criteria for Normoglycemia, Dysglycemia, and Hyperglycemia.
| Normoglycemia (stage 1) | Dysglycemia (stage 2) | Hyperglycemia (stage 3) | |
|---|---|---|---|
| FPG | <100 mg/dL (<5.6 mmol/L) | 100–125 mg/dL (5.6–6.9 mmol/L) | ≥126 mg/dL (≥7.0 mmol/L) |
| HbA1c | <5.7% (<39 mmol/mol) | a) 5.7–6.4% (39–47 mmol/mol) b) Increase ≥10% from last visit | ≥6.5% (≥48 mmol/mol) |
| OGTT | 2-h PG < 140 mg/dL (<7.8 mmol/L) | a) PG 140–199 mg/dL (7.8–11.0 mmol/L) at 120 min b) PG ≥ 200 mg/dL (≥11.1 mmol/L) at 30, 60, or 90 min | 2-h PG ≥ 200 mg/dL (≥11.1 mmol/L) |
| Random PG | – | – | Symptoms + PG ≥ 200 mg/dL (≥11.1 mmol/L) |
PG: plasma glucose; FPG: fasting plasma glucose; OGTT: oral glucose tolerance test.
The risk and rate of progression to stage 3 T1DM vary according to the type, number, and titer of beta-cell autoantibodies, and the age at seroconversion, estimated mainly from pediatric cohort studies.8–10
Screening for T1DM: considerations and recommendationsScreening strategiesFrom a methodological standpoint, 2 main screening strategies may be considered to identify individuals in presymptomatic phases of T1DM: An approach based exclusively on autoantibody detection, and approach combining genetic risk assessment, followed by autoantibody screening only in those with high genetic risk.11
Screening based exclusively on autoantibody detectionAutoantibody screening uses four types of antibodies—anti-GAD (GADA), anti-insulin (IAA), anti-IA2 (IA-2A), and anti-ZnT8 (ZnT8A)—as recommended by the ADA.7 Different detection methods are available: radioimmunoassay (RIA, considered the gold standard), luciferase immunoprecipitation system (LIPS), electrochemiluminescence (ECL), chemiluminescence, ELISA, antibody-detection-by-agglutination PCR (ADAP), among others. It is important that methods comply with the standards of the Islet Autoantibody Standardization Program (IASP). While available methods are validated and allow relatively simple screening from a technical and logistical standpoint, their sensitivity and specificity vary.12
When interpreting a positive autoantibody result—and thus considering autoimmunity as activated—international guidelines recommend following the “2 × 2 × 2 rule”: at least 2 positive autoantibodies, preferably determined with 2 different methods, and confirmed in 2 separate blood samples.13
Target population for T1DM screeningCurrently, 2 strategies are used for T1DM screening: a) Autoantibody detection in individuals with genetic risk/family history; b) Autoantibody detection in the general population. The first strategy has focused particularly on first-degree relatives (FDRs) of patients with T1DM, given their substantially higher genetic risk vs the general population. Indeed, the likelihood of developing T1DM in FDRs is approximately 10 times higher than in individuals without this background, including adult FDRs (parents and siblings). Several studies have shown that, even in adults, the presence of multiple autoantibodies confers a high risk of developing T1DM in the following years.13–15 Moreover, more than half of new T1DM cases are diagnosed in adulthood. These data support the need to include this population in screening programs.16–19 However, as nearly 90% of new T1DM cases occur in individuals without a family history,20 multiple pilot studies in neighboring countries are currently assessing the feasibility, acceptance, and outcomes of general population screening (particularly in children) to detect individuals with previously unrecognized risk. The ADA recommends screening in individuals with a family history of T1DM or known high genetic risk but does not currently recommend mass screening in the general population outside research protocols.7 Position statement: Screening in FDRs or individuals with known high genetic risk is supported by clinical evidence and by recommendations from specialist societies such as ISPAD and ADA. The development of pilot screening programs in the general pediatric population will allow assessment of their predictive performance, limitations, and cost-effectiveness in Spain and, ultimately, the feasibility of large-scale implementation in our setting.
- •
Screening is recommended in children with an affected FDR (parents, siblings), as the prevalence of T1DM in FDRs younger than 20 years (siblings: 6–7%, mother: 1.3–4%, father: 6–9%) is 15 times higher vs the general population.15,21
- •
Drawing 2 blood samples during childhood seem to be the most cost-effective strategy: the first between ages 2–3 years. If negative, it should be repeated between ages 6–8 years, or 4 years after the initial test regardless of age at first screening.
- •
The optimal timing for detecting autoimmunity during adolescence (ages 10–18 years) is 1 test at age 10, or 2 tests at ages 10 and 14. Screening after age 14 is not recommended.
- •
Between ages 18 and 45 years, a single autoantibody test is advised. If negative, repetition is not recommended.
- •
A negative result indicates a very low immediate risk of progression to T1DM, but does not exclude the possibility of future development.
Information about screening should follow basic principles:22 the right not to know, respect for autonomy (individuals must make their own decisions), and equality of access to health care.
Follow-up and monitoring of individuals with positive autoimmunity markersA positive autoantibody screening result should be accompanied by regular follow-up to monitor disease progression and enable early intervention.2 It is essential to have monitoring methods that can detect—early and accurately—when a patient has progressed to stage 3. Ideally, these monitoring methods should be minimally invasive, easy to use, and rapid because, according to some studies, adherence to certain current follow-up methods is suboptimal.23
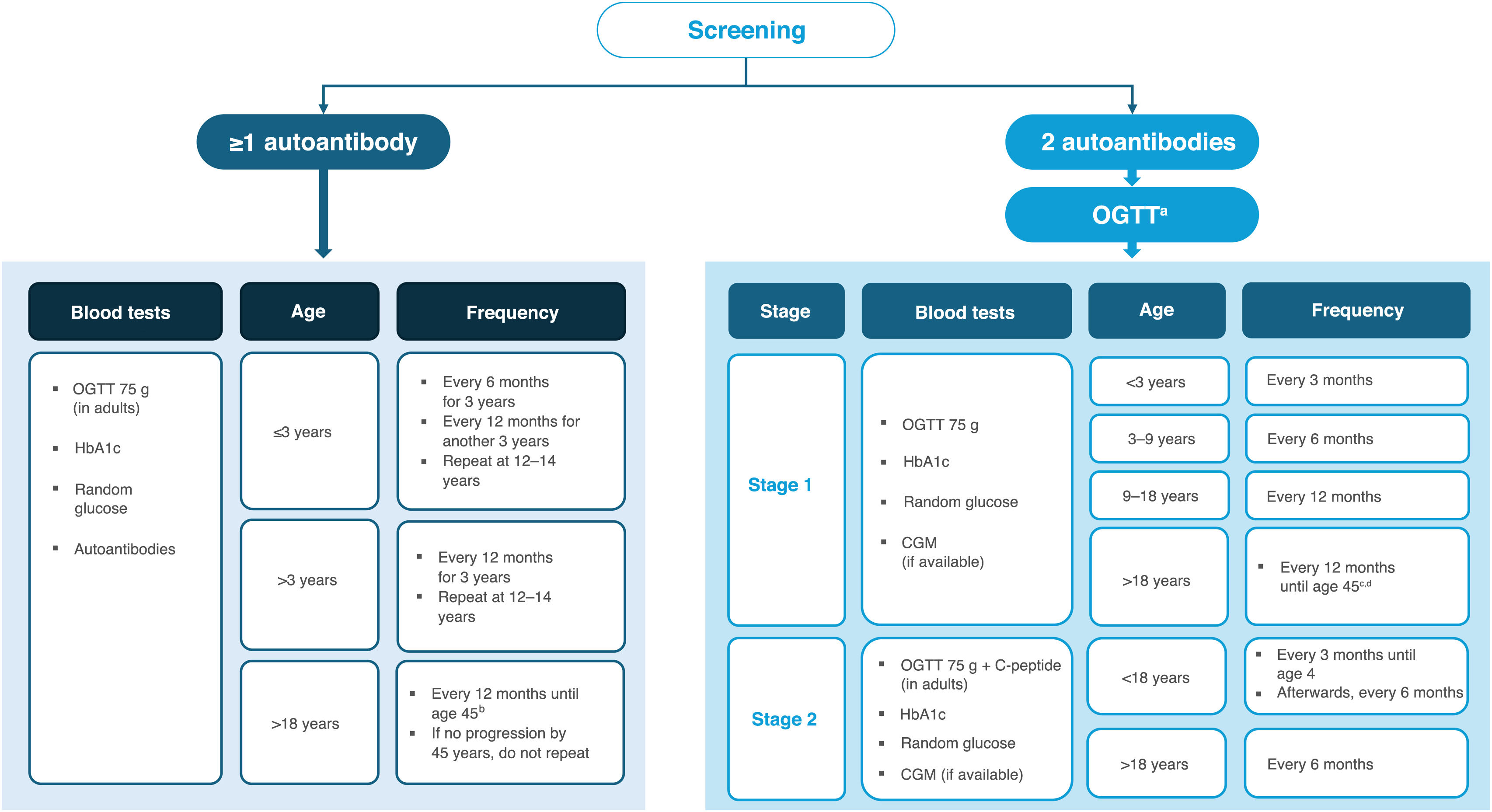
Recommendations for follow-up of individuals with autoimmunityFollow-up and monitoring strategies depend on age and on the number of beta-cell autoantibodies. Such strategies are described in detail in the 2024 consensus guideline by major scientific societies,2 which is considered appropriate in our context (Fig. 2). It is recommended to offer glycemic assessments and ongoing follow-up to individuals 2 test positive for one or more beta-cell autoantibodies.2,3
Follow-up and monitoring recommendations for high-genetic-risk FDRs with positive beta-cell autoimmunity.
a HbA1c, capillary/venous glucose, and CGM can be alternatives (primarily in children) when the OGTT is not feasible. The pediatric OGTT uses 1.75 g of glucose per kilogram of body weight, up to a maximum of 75 g.
b If not an FDR/no additional risk factors, every 3 years.
c From age 45 years, metabolic follow-up may continue according to established primary-care T2DM screening.
d If not an FDR/no additional risk factors and no progression over 5 years, every 2 years.
Pregnant women with positive autoantibodies should undergo an OGTT and HbA1c (and, if available, consideration of continuous glucose monitoring [CGM]) at confirmation of pregnancy and again at 24–28 weeks, in accordance with standardized gestational diabetes screening protocols. Those diagnosed with T1DM should be evaluated in the immediate postpartum period and prior to hospital discharge by an endocrinologist to determine the need for continued insulin therapy. Whether or not clinical T1DM is diagnosed, pregnant women with autoantibodies should be monitored postpartum for 6–12 months to assess insulin requirements.2 Thereafter, if there is no progression to stage 3, follow-up should proceed as for autoantibody-positive adults.
Recommendations for Patient/Family educationAfter confirming stage 1 or stage 2 T1DM, patients/caregivers need education on disease progression and appropriate support. Referral to a specialized center/clinician (Pediatric Endocrinology Units or Endocrinology and Nutrition Services) is recommended. Health professionals should explain the probabilities of progression to stage 3 based on the number and type of autoantibodies and glycemic status, to support patients/caregivers in understanding and accepting the risk of progression to clinical T1DM. Written instructions should be provided, including contact information and locally available urgent-care pathways in case of T1DM symptoms and/or hyperglycemia.
When patients eventually progress to stage 3 T1DM, the foundational pillars of good disease management should be conveyed: healthy nutrition, an active lifestyle, insulin therapy, and periodic glucose monitoring.24
Evaluating the impact of a T1DM screening and follow-up programOne of the most common ways to evaluate follow-up and program impact is to assess whether early diagnosis reduces the frequency of diabetic ketoacidosis (DKA) at diagnosis. Several studies—primarily in pediatric populations—have shown that participation in a preclinical follow-up program reduces DKA at diagnosis,25 and is associated with shorter lengths of stay, likely due to earlier diagnosis and less overall deterioration at presentation.26 DKA is not only a serious risk per se; it is also a potential risk factor for the development of diabetes complications, as it is associated with difficulty achieving long-term glycemic targets.27
Regarding costs, no data are yet available in Spain; therefore, cost-effectiveness analyses are needed in ongoing pilot studies among relatives13 and in the general population.
Proposed impact indicatorsAs specific indicators of the impact of launching a family-based screening program with systematic monitoring (analyzed separately for pediatric and adult populations), the following are proposed:
- –
Proportion of individuals “at risk” (single positive autoantibody) among all screened first-degree relatives (FDRs).
- –
Proportion diagnosed with stage 1 T1DM among all screened FDRs.
- –
Proportion diagnosed with stage 2 T1DM among all screened FDRs.
- –
Proportion diagnosed with clinical T1DM (stage 3) among all screened FDRs.
- –
Proportion diagnosed with clinical T1DM (stage 3) presenting as DKA among all individuals identified as stage 1 or 2 through the FDR screening program.
- –
Annual incidence rate of DKA in the area where the screening program is implemented (compared with a non-screened population).
- –
Annual incidence rate of severe DKA in the implementation area (vs a non-screened population).
T1DM screening can be controversial because a positive result does not predict precisely when the disease will appear: in children, in whom the evidence is strongest, 53% of those with two autoantibodies (stage 1 or 2) at 5 years are insulin-dependent (stage 3), rising to 82% at 10 years.13 In adults, progression between stages is slower and more uncertain, complicating the design of educational and therapeutic intervention programs.
Moreover, recent clinical trials in presymptomatic T1DM have shown promising benefits,28 although they do not definitively prevent stage-to-stage progression, and published findings are not yet applicable to all stages and populations under screening. Since most T1DM cases arise without a family history, a significant reduction in DKA would require population-level screening, which necessitates prior evaluation of feasibility and benefit–risk in pilot studies. Finally, training health care professionals and stakeholders is essential to ensure appropriate follow-up and patient education.
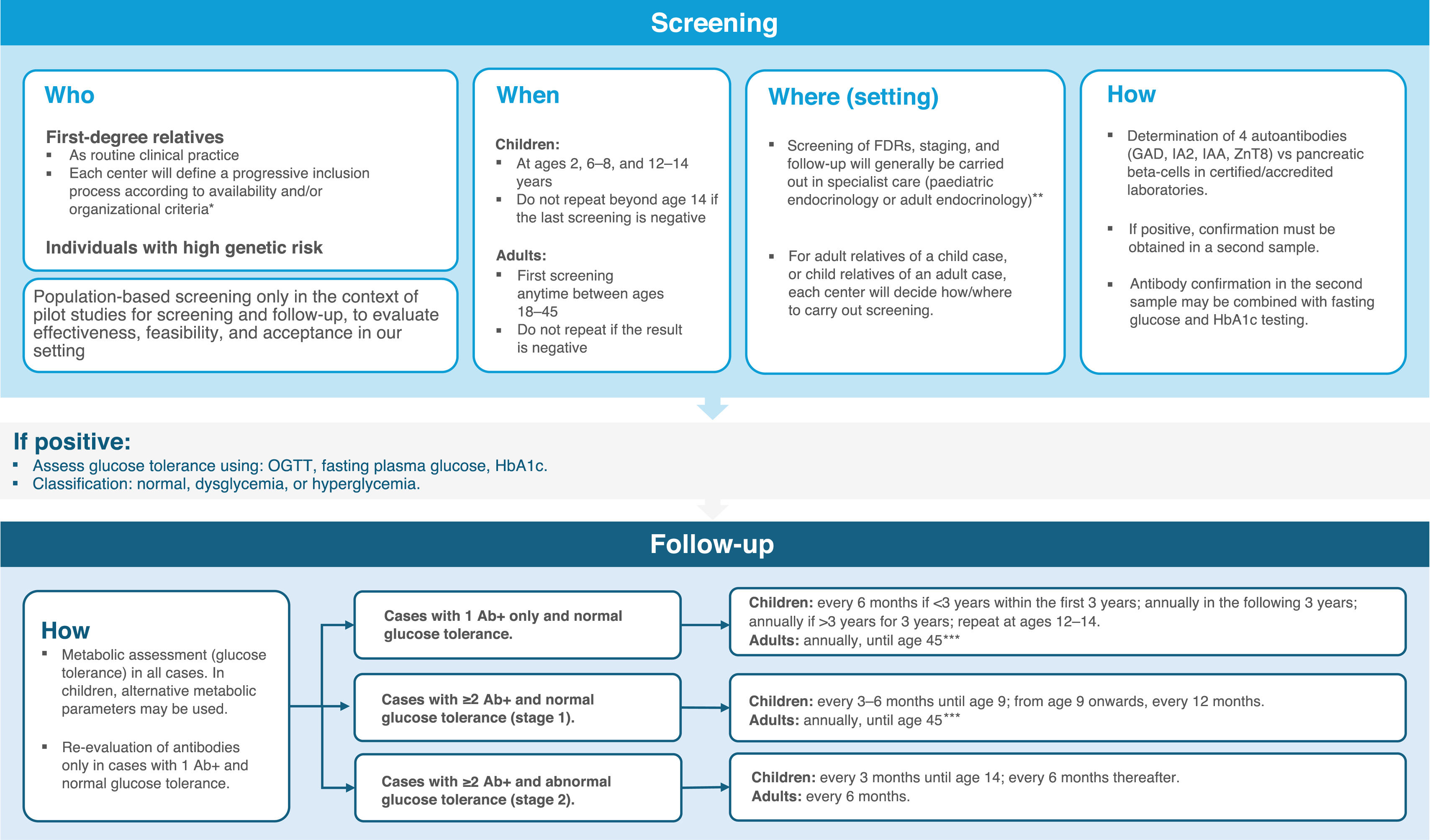
Summary of recommendations and conclusionsRecommendations for screening and follow-up in early T1DM stages are summarized in Fig. 3.
Recommendations for screening and follow-up of presymptomatic T1DM.
* The presymptomatic T1DM screening and follow-up strategy should ensure minimum uniform standards across Spain, tailored to the characteristics and needs of each region and health service to ensure equity.
** In general-population pilot programs, screening may be performed in family pediatrics teams; however, follow-up and staging should be undertaken by specialized teams.
*** If not an FDR/no additional risk factors and no progression over 5 years, every 2 years. From age 45 years, metabolic follow-up may continue according to established primary-care T2DM screening.
The protocol presented here should be adapted to available resources and to the specific circumstances of each center or region and kept up to date as new scientific findings and international recommendations emerge.
FundingThe Spanish Society of Endocrinology and Nutrition, the Spanish Diabetes Society, and the Spanish Society of Paediatric Endocrinology funded the medical writing services provided by Springer. The authors did not receive any direct or indirect financial compensation for the development of this work, which was supported by the above-mentioned scientific societies.
María Asunción Martínez-Brocca is principal investigator in studies sponsored by Diamyd Medical AB and Sanofi and has participated in paid activities for Novo Nordisk and Sanofi.
Virginia Bellido has received professional fees (consulting, research, or lectures) from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Esteve, MSD, Novo Nordisk, and Sanofi.
Roque Cardona-Hernandez is principal investigator in studies sponsored by Diamyd Medical AB, Novartis, and Sanofi, and has received honoraria for lectures or scientific advising from Abbott, Dexcom, Medtronic, Novo Nordisk, and Sanofi.
Luis Castaño has participated in scientific activities funded by Novo Nordisk, AstraZeneca, and Sanofi.
Ignacio Conget has collaborated with Abbott, Medtronic, Dexcom, Lilly, Sanofi, Novo Nordisk, Bayer, and Ascensia.
Alberto Fernández has participated in educational sessions and discussion forums organized by Novo Nordisk, Lilly, AstraZeneca, Abbott, and Boehringer.
Ana Lucía Gómez Gila has received professional fees from Novo Nordisk Pharma, Sandoz Farmacéutica, and Sanofi Aventis.
Isabel Leiva-Gea is principal investigator in studies sponsored by Diamyd Medical AB and has participated in paid activities for Abbott, Dexcom, Medtronic, Novo Nordisk, Eli Lilly, Sanofi, and Biomarine.
Dídac Mauricio has received consulting and lecture honoraria from AB-Biotics, Abbott, Almirall, Amarna, Amgen, AstraZeneca, Ferrer, Gilead, Lilly, Medtronic, Menarini, MSD, Novo Nordisk, and Sanofi.
The authors thank Anabel Herrero on behalf of Springer Health care for assistance in preparing the manuscript, as well as the support of the scientific societies represented by the authors.
This article was jointly prepared by Endocrinología, Diabetes y Nutrición, Endocrinología, Diabetes y Nutrición (English Edition), Anales de Pediatría, Anales de Pediatría (English Edition), and jointly published by Elsevier España S.L.U. The article are identical except for minor stylistic and spelling differences consistent with each journal’s style. Either citation may be used when referencing this article.