Pulmonary sequestration is a condition in which a segment of the lung has no identifiable communication with the tracheobronchial tree and receives an anomalous vascular supply from the systemic arteries, resulting in a ventilation-perfusion mismatch. It accounts for 0.15% to 6.4% of pulmonary malformations, and it carries a risk of late, although infrequent, malignant transformation, as there have been reports of adenocarcinoma developing in the involved tissue. There are two forms: intralobar, when the mass is located within the pleura that surrounds the rest of the lung and the venous drainage is to the left atrium, found in 75% of cases and manifesting at older ages with recurrent pneumonias or haemoptysis. Extralobar, when the mass has a separate pleura and the venous drainage is to a systemic vein, generating a left-to-right shunt that can lead to pulmonary hypertension from an early age, and frequently associated with other congenital anomalies.1
Until recently, treatment consisted of surgical resection of the involved tissue with ligation of the feeding vessels. At present, there is scientific evidence supporting the use of endovascular occlusion as a definitive treatment option.2 Coils have been the most widely used devices, but the use of Amplatzer vascular occluders has also been described in older children and adults.3
We present a case series of infants treated with Amplatzer Piccolo™ occluders approved for closure of patent ductus arteriosus in preterm infants.
Three patients (Table 1) were admitted with a diagnosis of pulmonary sequestration and clinical manifestations of pulmonary hypertension. The CT angiography scan evinced the presence of aberrant vessels stemming from the abdominal aorta and entering the right lower lobe, which in 2 patients were also associated with partial anomalous pulmonary venous return to the inferior vena cava with scimitar syndrome. Endovascular occlusion was chosen as the treatment option and the use of the use of Amplatzer Piccolo™ occluders was considered due to the weight of the patients and with the aim of decreasing the risk of complications associated with an arterial approach.
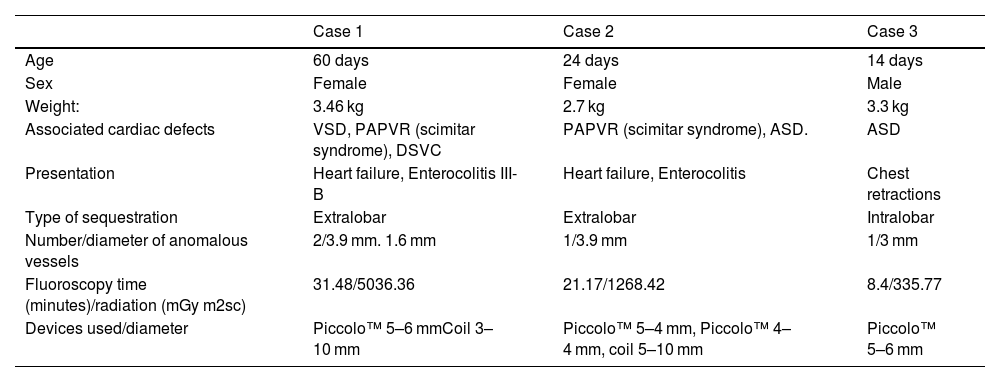
Clinical and angiographic features and devices used in infants with pulmonary sequestration.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age | 60 days | 24 days | 14 days |
| Sex | Female | Female | Male |
| Weight: | 3.46 kg | 2.7 kg | 3.3 kg |
| Associated cardiac defects | VSD, PAPVR (scimitar syndrome), DSVC | PAPVR (scimitar syndrome), ASD. | ASD |
| Presentation | Heart failure, Enterocolitis III-B | Heart failure, Enterocolitis | Chest retractions |
| Type of sequestration | Extralobar | Extralobar | Intralobar |
| Number/diameter of anomalous vessels | 2/3.9 mm. 1.6 mm | 1/3.9 mm | 1/3 mm |
| Fluoroscopy time (minutes)/radiation (mGy m2sc) | 31.48/5036.36 | 21.17/1268.42 | 8.4/335.77 |
| Devices used/diameter | Piccolo™ 5–6 mmCoil 3–10 mm | Piccolo™ 5–4 mm, Piccolo™ 4–4 mm, coil 5–10 mm | Piccolo™ 5–6 mm |
ASD: atrial septal defect; DSVC: duplicated superior vena cava; PAPVR: partial anomalous pulmonary venous return; VSD: ventricular septal defect.
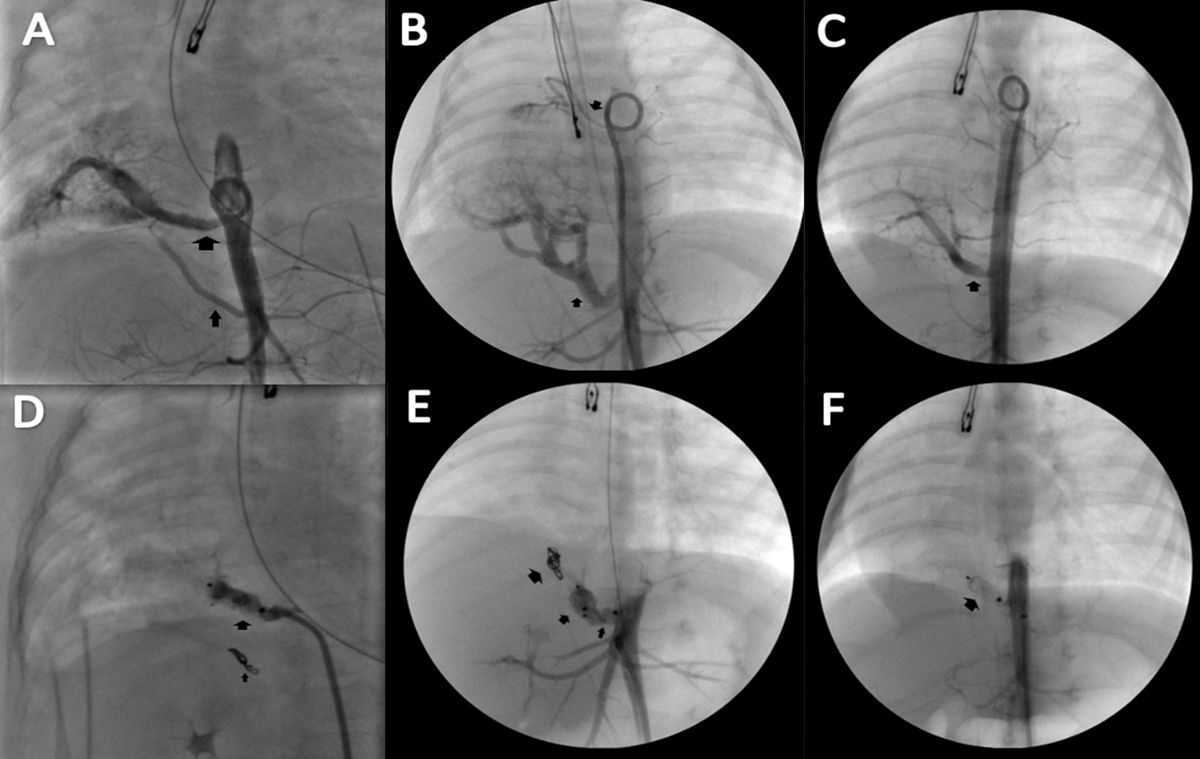
After obtaining informed consent from the parents and with the patient under general anaesthesia, femoral access was gained for placement of 4 F delivery catheters, with administration of a single dose of heparin sodium (100 U/kg/dose) and performance of aortography to identify the aberrant vessels (Fig. 1A, B, C). The latter were selectively catheterised and, using the Amplatzer™ TorqVue™ low profile delivery system 4 F, the Amplatzer Piccolo™ (AGA Medical Corporation) occluders were deployed for embolization of the feeding vessels (Fig. 1D, E, F). In addition, coils were placed in one vessel of smaller calibre.
Pre- and post-occlusion angiographs of the feeding vessels of pulmonary sequestration.
(A), (B), (C): Baseline aortograms of cases 1, 2 and 3. The arrows indicate the feeding vessels arising from the aorta that supplied the pulmonary sequestration.
(D), (E), (F): Post-occlusion angiographs of the feeding vessels of cases 1, 2 and 3. The arrows indicate the implanted devices.
In one female patient, the catheterization procedure was repeated 4 weeks later to assess the closure of the ventricular septal defect, evincing persistence of residual flow in a feeding vessel that was subsequently occluded through the delivery of 2 additional coils. All patients exhibited clinical and radiological improvement with resolution of the manifestations of pulmonary hypertension.
There were no complications of catheterization either in the immediate postoperative period or in the 2 years of follow-up.
Historically, the treatment of pulmonary sequestration has been based on surgical resection of the anomalous tissue and ligation of the feeding vessels. The combination of embolization plus surgical resection and in some cases observation has also been described.4 At present, there is evidence in support of the use of endovascular occlusion as a definitive treatment option, as there are data suggestive of the involution of pulmonary sequestration following embolization. However, the use of this technique has been proposed for older children due to the risk of vascular complications in very young children.2
Endovascular occlusion of pulmonary sequestration was first described by Rothman in 1993,5 and coils have been the devices most widely used until now, but most cases require multiple coils, with a high frequency of residual shunt requiring multiple reinterventions. On the other hand, the use of Amplatzer™-type devices, such as vascular occluders with 5 F delivery systems, has been described in older children and adults.3
The Amplatzer Piccolo™ occluder was approved by the United States Food and Drug Administration (FDA) for closure of patent ductus arteriosus and its use for treatment of scimitar syndrome in a boy aged 2.5 years was recently reported.6 It is a self-expanding device made of nitinol with a central waist diameter ranging from 3 to 5 mm, a length of 2, 4 or 6 mm and retention discs measuring 4 to 6.5 mm. It uses a 4 F delivery system, which reduces the risk of vascular complications associated with the use of larger delivery catheters in small children.
This is the first case series describing the use of the Amplatzer Piccolo™ for endovascular treatment of pulmonary sequestration in infants. It demonstrates that this is a feasible and safe technique that may be used in infants and newborns.