Primary ciliary dyskinesia (PCD) and cardiospondylocarpofacial (CPFC) syndrome belong to the group of rare diseases owing to their very low prevalence in the population (1/7554 for PCD and <1/1 000 000 for CPFC syndrome). We present the case of a girl with an initial genetic diagnosis of SCECF who, due to worsening respiratory manifestations and the development of bronchiectasis received a diagnosis of SCPD at a later time.
The girl, currently aged 3 years, presented with a peculiar phenotype at birth (bulging forehead, flat philtrum, low-set ears, broad neck) and symptoms including generalised hypotonia, feeding difficulty, tachypnoea, chronic rhinitis and respiratory acidosis. She remained hospitalised for the first 3 months post birth, during which partial right upper lobe atelectasis was detected on the chest X-ray and foramen magnum stenosis with compression of the cervicomedullary junction on the brain MRI, requiring surgery at age 2 months.
At this time, the evaluation was extended with performance of echocardiography, which detected mild tricuspid regurgitation, and genetic testing with trio exome sequencing to assess for neurologic disorders, with identification of a de novo likely pathogenic variant (c.821G>A) in the MAP3K7 gene associated with CPFC syndrome that encompasses growth retardation, facial dysmorphic features, hypotonia, feeding difficulties, heart disease and malformations involving the cervical spine.1
In the first year of life, she exhibited chronic respiratory symptoms including daily wet cough and rhinitis and recurrent respiratory tract infections manifesting with bronchitis, cold symptoms and bilateral chronic serous otitis media. The salient findings of the physical examination were persistent abnormal lung sounds with bilateral wet rales, chest retractions and tachypnoea with a normal oxygen saturation. She also had oropharyngeal dysphagia diagnosed by video fluoroscopy, with impaired swallowing and poor weight gain (2nd percentile), due to which she required tube feeding until age 5 months.
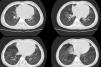
Due to suspicion of chronic aspiration syndrome secondary to dysphagia possibly associated with gastro-oesophageal reflux (the latter subsequently ruled out by pH-metry), a CT scan of the lungs was performed that revealed subsegmental atelectasis in the middle lobe and lingula with small areas of bronchiectasis and mild peribronchovascular thickening predominantly involving the lower lobes (Fig. 1). These findings motivated performance of flexible bronchoscopy, which evinced an abundance of mucus throughout the bronchial tree, and with collection of a bronchoalveolar lavage sample that was positive for Haemophilus influenzae. The treatment consisted of inhaled and nasal budesonide, nebulised hypertonic saline solution, respiratory physical therapy and oral azithromycin 3 days a week.
The respiratory problems in the patient, given the few respiratory complications associated with CPFC syndrome, motivated the consideration of alternative diagnoses manifesting with bronchiectasis, such as cystic fibrosis or PCD. Although the results of newborn screening with immunoreactive trypsinogen were normal, a sweat test was conducted, which also had normal results. The trio exome sequencing results were re-evaluated, with identification of the homozygous variant c.246+1G>C in the ODAD4 or TTC25 gene, associated with PCD, that encodes the outer dynein arm and results in severe impairment of ciliary movement.2
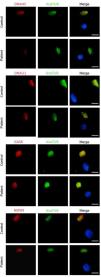
The patient was referred to the PCD reference unit, where the diagnosis was confirmed by the absence of ciliary movement (static cilia) on high-speed video microscopy, the decreased concentration of nasal nitric oxide (nNO) of 13.2 nL/min during tidal breathing and absence of DNAH5 staining in most ciliated cells on immunofluorescence (Fig. 2).
Immunofluorescence analysis of ciliary axoneme proteins in a specimen of ciliated epithelium obtained from the patient and a control specimen. Subcellular location of ciliary axoneme proteins DNAH5, DNALI1, GAS8 and RSPH9 (in red), and acetylated α-tubulin (AcαTUB, in green). The third column shows the merged channels with the nuclei stained with DAPI (in blue). DNAH5 is absent from the ciliary axoneme of the patient’s sample compared to the control sample. Scale bar: 5 μm.
Primary ciliary dyskinesia results from a structural defect in ciliary cells present in the respiratory system and gonads resulting in impaired ciliary movement and decreased mucociliary clearance, which in turn increases the risk of respiratory infection. In most cases, it follows an autosomal recessive pattern of inheritance, with more than 50 variants reported to date,3 and is associated with respiratory tract and otic, nasal and laryngeal manifestations, sterility, ectopic pregnancy and, in 50% of cases, situs inversus with dextrocardia. On imaging, it manifests with peribronchial thickening with atelectasis or bronchiectasis.4
The diagnosis is based on screening with nNO (usually decreased) and confirmed by electron microscopy (ultrastructural abnormalities), immunofluorescence, high-speed video microscopy (ciliary beat pattern and frequency) and genetic testing.5 The management is based on the improvement of mucociliary clearance by means of respiratory physical therapy, aerobic exercise and mucolytic agents (nebulised hypertonic saline solution); control of respiratory infections (oral or intravenous treatment in respiratory exacerbations and nebulised antibiotherapy) and anti-inflammatory drugs, such as oral azithromycin.6
In conclusion, we want to underscore the importance of ruling out the presence of additional diseases in all patients with a confirmed syndrome diagnosis whose course of disease or clinical features do not fit the typical phenotype of the disease, as was the case in our patient.
CRediT authorship contribution statementAll authors participating in the writing of the manuscript.