Near-infrared spectroscopy (NIRS) is a non-invasive continuous monitoring technique that has been employed successfully in some studies to monitor the cerebral and splanchnic regions in preterm infants.1–4 Spatially resolved spectroscopy uses near-infrared light (600–900 nm) to detect the presence of chromophores, especially haemoglobin, but also cytochrome aa3 and myoglobin. Near-infrared spectroscopy measures venous, arterial and capillary oxygenation, therefore reflecting the delivery of oxygen to tissues and its consumption. It allows early detection of problems in regional oxygenation in different tissues, even before injury and damage to the tissue takes place. A majority of studies have found an association between lower splanchnic oxygen saturations in premature infants with a history of intrauterine growth restriction and with the risk of necrotizing enterocolitis in infancy.1–3
Neonatal gastric perforation is a rare surgical emergency that amounts to approximately 7% of the total cases of gastrointestinal perforation in the neonatal period. It is associated with a high morbidity and mortality ranging from 41% to 80%. Its aetiology is multifactorial and includes clinical conditions and iatrogenic trauma to the gastrointestinal tract. Also, the use of steroids or severe physiological stress may increase gastric acid secretion, resulting in production of stress ulcers. However, the pathophysiology of this lesion remains unknown. The clinical presentation is frequently nonspecific. The clinical signs and symptoms include abdominal distention, feeding intolerance, decreased activity, haemodynamic changes and respiratory distress. The diagnosis requires confirmation by an abdominal radiograph with visualization of pneumoperitoneum and, in some cases, a pneumoscrotum.4–6
In this article, we describe the role of continuous abdominal monitoring with NIRS in the neonatal intensive care unit (NICU) and its contribution to the early prediction of gastrointestinal complications.2,3
We present the case of a premature infant delivered at 30 weeks’ gestation by caesarean section with a birth weight of 880 g. The infant was admitted to the NICU for respiratory support. Intrauterine growth restriction had been detected during gestation. The mother received a full course of antenatal steroids and magnesium sulphate. The 1-min and 5-min Apgar scores were 7 and 8, respectively, and the umbilical cord blood gas analysis revealed an arterial pH of 7.22. The infant was transferred to the NICU receiving continuous positive airway pressure (CPAP) through nasal prongs with an inspired fraction of oxygen (FiO2) of 0.21.
The patient had mild respiratory distress but remained stable without need of increasing the FiO2 or surfactant replacement therapy. He remained on nasal intermittent mandatory ventilation from day 1 to day 4 post birth, at which point he was switched to CPAP with nasal prongs. He only required increases in FiO2 occasionally during handling/procedures. Caffeine was also administered from 24 h post birth. Prophylactic antibiotherapy was initiated due to severe neutropenia (neutrophil count <500 cells/µL) soon after birth. Trophic enteral feeding with human donor milk was initiated at 24 h post birth. At 48 h, the physician in charge diagnosed hemodynamically significant ductus arteriosus, which was treated successfully with a 3-day course of paracetamol, as ibuprofen was contraindicated due to thrombopenia.
The infant passed meconium 48 h post birth with saline enemas. In the days that followed, bowel movements were only achieved with further stimulation with saline enemas. We ought to note that the patient did not tolerate enteral feeding even for a minimum volume of with breast milk. Suspicion of meconium plug syndrome (MPS) led to treatment with both oral and rectal N-acetylcysteine, initiated on day 4 post birth. Abdominal radiographs and an ultrasound examination ruled out additional complications of MPS. The patient was under continuous NIRS monitoring with the INVOS 5100 system (Medtronic®, Fridley, Minnesota, USA) and the Neonatal Oxy-Alert NIRS Sensor (Medtronic®, Fridley, Minnesota, USA). The patient had a splanchnic oxygenation sensor placed on the right-side flank from day 1 of life.
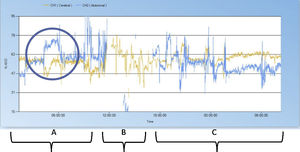
From the first 24 h of life, the cerebral and splanchnic NIRS values had been stable, without significant changes out of the normal range for the patient’s age (55–65). Suddenly, on day 5, the infant developed hyperglycaemia, tachycardia, arterial hypertension and increased abdominal distension. An urgent radiograph revealed the presence of pneumoperitoneum. The NIRS register showed a sharp decrease in regional cerebral oxygenation (>25%) with a simultaneous significant increase in the regional somatic oxygenation (>25%) (Fig. 1) in the previous 6 h. A subsequent review of the recordings showed that both events coincided in time with the development of gastrointestinal perforation, which was only suspected 6 h later based on the clinical and radiographic features.
Record of regional oxygenation (rSO2). In yellow, cerebral rSO2 and in blue, abdominal rSO2. (A) NIRS tracing 12 h before surgery with significant increase in abdominal rSO2 and a decrease in cerebral rSO2 coinciding with the timing of gastric perforation. (B) NIRS tracing during surgery (4 h). (C) NIRS tracing 12 h after laparotomy and repair of gastric perforation.
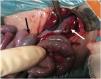
Treatment with N-acetylcysteine treatment with enemas was unsuccessful. An emergency upper median laparotomy was performed, revealing the presence of bile and air in the peritoneum. The surgical findings were gastric perforation 2 cm in diameter in the greater curvature extending toward the fundus with necrotic changes in the surrounding tissue. The stomach was surgically repaired. The abdominal exploration also confirmed meconium obstruction of the ileum. An infusion of saline was administered through an enterotomy in the terminal ileum for manual removal of the impacted meconium. Despite the removal of meconium during the surgery, the patient did not exhibit improvement in the intestinal transit and underwent an ileostomy at 29 days post birth.
Gastric perforation is an infrequent surgical emergency in the neonatal population. Our patient presented with feeding intolerance, hyperglycaemia, and abdominal distension. Subsequently, an urgent radiograph detected pneumoperitoneum. These clinical signs and symptoms are the most frequently described in previous studies.4,5 Lastly, the abdominal surgery revealed gastric perforation and meconium ileus.
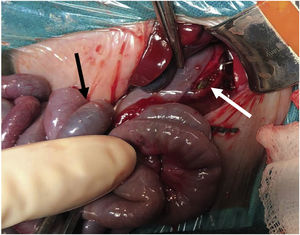
We suspected that the gastric perforation of this patient had a multifactorial aetiology including low birth weight, prematurity, increased intragastric pressure due to meconium impaction and use of non-invasive mechanic ventilation. We also suspected involvement of iatrogenic trauma associated with the orogastric tube (Fig. 2). Each of these factors involved in the development of gastric perforation has been described previously.4
Among the salient findings of the retrospective interpretation of the NIRS recording, we ought to highlight a simultaneous elevation of the regional abdominal oxygen saturation and decrease in the regional cerebral oxygen saturation that we suspected coincided with the timing of gastric perforation. However, the oxygen saturation measured by pulse-oximetry remained unchanged, which implied an increase in cerebral oxygen extraction. The sonographic findings excluded other possible causes of the regional cerebral oxygen saturation drop. There was no evidence of ductal activity or intracranial haemorrhage. Therefore, we suspected that the increase in cerebral oxygen extraction was associated to pain and stress secondary to the gastric perforation, which would also explain the presence of moderate hyperglycaemia, tachycardia and arterial hypertension.
Extremely preterm infants are at risk of several complications. However, the signs and symptoms are often delayed, and the fragile condition of these patients places significant restrictions on the performance of possible imaging procedures. Near-infrared spectroscopy is a non-invasive tool for real-time continuous monitoring that provides helpful information to clinicians.1–3 Unfortunately, most studies on NIRS are retrospective, and only a few have data collected prospectively.1–3 However, NIRS allows early detection of changes in tissue oxygenation in specific regions even before the injury becomes significant and the clinical manifestations apparent, which makes it possible for clinicians to deliver preventive and/or therapeutic interventions.1–3
Near-infrared spectroscopy detects changes in cerebral and somatic perfusion pressure, such as hypoperfusion states that entail decreased oxygenation due to a reduction in the oxygen supply. Haemodynamically stable patients may also exhibit abnormalities or changes in tissue oxygenation secondary to an increase in tissue oxygen consumption (stress, pain, sepsis, hyperthermia, sedation, seizures…) and changes in the partial pressure of oxygen and haemoglobin levels.
Our study is relevant in that it demonstrates that there are significant changes in regional oxygen saturation (NIRS) a few hours before the clinical diagnosis. It also demonstrates differences in oxygenation associated in time with gastric perforation, an infrequent acute pathology, and other and more frequent insidious gastrointestinal diseases, such as necrotising enterocolitis (NEC). A study on NEC found that decreases in regional cerebral oxygen saturation predated the clinical diagnosis by several days.1–3 Nevertheless, in this case of gastric perforation, regional oxygenation changes happened quickly and showed an unexpected increase. We still do not know the reason for this phenomenon. One possible explanation is the difference in the pathophysiology of these 2 intestinal diseases.
To optimise the information yield of NIRS, it is necessary for nurses to report any significant changes reflected in the monitor’s display. Thus, continuous training of the nursing and medical staff is of the essence.
In conclusion, NIRS monitoring of abdominal regional oxygen saturation may provide a new approach to the early diagnosis and treatment of gastric and intestinal perforation in the neonatal population.
FundingA. Solaz-García, is a research fellow of RETICS funded by the grant PN 2018-2021, Instituto de Salud Carlos III-General Vice Directorate General for Research Assessment and Promotion (Spain). European Regional Development Fund (ERDF) grant, reference RD16/0022, awarded to M Vento.
We want to give special thanks to the parents of the neonate, who gave their consent to use the images. We also thank all the professionals that care for newborn infants in the NICU of the Hospital Universitario y Politécnico La Fe in Valencia, Spain.
Please cite this article as: Torres-Martínez E, Sáenz-González P, Rodríguez-Caraballo L, Driller C, Vento M, Solaz-García Á. Uso de la espectroscopia cercana al infrarrojo en las perforaciones gástricas en neonatología. An Pediatr (Barc). 2022;96:256–258.