The rational use of antibiotics (ATB) implies that patients receive those adequate for their clinical needs, in correct doses according to their individual conditions, during an adequate period of time, and at the lowest cost for them and their community. The highest rate of ATB abuse occurs during the perinatal period, despite the fact that there is evidence of multiple short- and long-term negative effects. Furthermore, this abuse is associated with increased costs of medical care.
ObjectiveTo update and report the evidence on the use, abuse, and adverse effects of ATB in perinatal medicine, and possible measures to prevent them, and thus improve health care outcomes and costs.
MethodsA review and analysis was performed from the literature related to the use of ATB in perinatal medicine up to February 2020.
ResultsATB abuse in perinatal medicine ranges from 50% to 70%, with even higher rates in some neonatal centres. Adverse effects include death, increased microbial resistance, along with microbiome abnormalities and dysbiosis that lead to serious life-long complications such as infections, allergies, autoimmune disorders, gastrointestinal disorders, arthritis, asthma, obesity, and perhaps cancer. Preventing and reducing the abuse of ATB would lead to better health and to significant savings in the health sector. In only 4 neonatal intensive care units, with 1000 admissions per year, savings are estimated at US$230,000 per year.
ConclusionThe need to optimise the use of ATB in perinatal medicine has never been more urgent.
El uso racional de antibióticos (ATB) implica que los pacientes reciban ATB adecuados a sus necesidades clínicas, en dosis correctas según sus condiciones individuales, durante el tiempo adecuado y al menor costo para ellos y su comunidad. La mayor tasa de abuso de ATB ocurre durante el período perinatal, a pesar que existe evidencia de múltiples efectos negativos a corto y largo plazo. Además, este abuso se asocia con incrementos en los costos de la atención médica.
ObjetivoActualizar y reportar la evidencia sobre el uso, abuso y efectos adversos de ATB en medicina perinatal y las posibles medidas para prevenirlos y, de este modo, mejorar la calidad de los cuidados, los resultados y los costos.
MétodosRevisión y análisis de la literatura relacionada al uso de ATB en perinatología hasta febrero 2020.
ResultadosEl abuso de ATB en perinatología oscila entre 50% y 70% y aún más en algunas unidades neonatales. Los efectos adversos incluyen morbilidades agudas, muerte, aumento de resistencia microbiana, alteraciones del microbioma y disbiosis asociadas a complicaciones graves a lo largo de la vida, como infecciones, alergias, trastornos autoinmunes, enfermedades gastrointestinales, artritis, asma, obesidad y tal vez cáncer. Prevenir y disminuir el uso indebido de ATB conducirá a mejorar la salud y a ahorros significativos en el sector sanitario. En solamente 4 UCIN con 1000 admisiones anuales, el ahorro se estima en 230.000 dólares por año.
ConclusiónLa necesidad de optimizar la utilización de ATB en la medicina perinatal nunca ha sido más urgente.
Antibiotics save lives, but unfortunately, they are often used incorrectly. The Conference of Experts on the Rational Use of Drugs of the World Health Organization (WHO) (OMS) (Nairobi 1985) defined rational use as follows: Rational use of drugs requires that patients receive medicines appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost to them and their community. The WHO estimates that more than half of all drugs are prescribed incorrectly. The most important problems are the use of too many drugs per patients (polytherapy) and the inappropriate use of antimicrobial drugs.1 The abuse of antibiotics is a global problem with dire consequences for health and the economy. In perinatal medicine, irrational antibiotic use is important for several reasons: pregnant women and the foetus/infant go through a highly vulnerable period during gestation and in the neonatal period, and it is during this time and in the first year post birth that the abuse of antibiotics is highest. Between 55% and 65% of pregnant women and more than 70% of newborns admitted to some neonatal intensive care units (NICUs) receive antibiotics during the hospital stay.2 Antibiotic overuse is associated with the emergence of antimicrobial resistance, alterations in the microbiome and dysbiosis, which in turn have been associated with longer lengths of stay, increased mortality and various diseases including infections, asthma, obesity, diabetes, atherosclerosis and autoimmune disorders, among others, throughout the lifespan. The establishment of interdisciplinary teams to collaboratively design and implement effective interventions aimed at optimising antibiotic use in perinatal medicine is a challenge that requires significant effort but could yield extraordinary rewards.
Antibiotic overuse during pregnancyMost pregnant women use some form of medication during gestation, and antibiotics are among the most frequently prescribed drugs.3 Thus, it is frequent for a majority of foetuses today to have been exposed to antibiotics before birth.
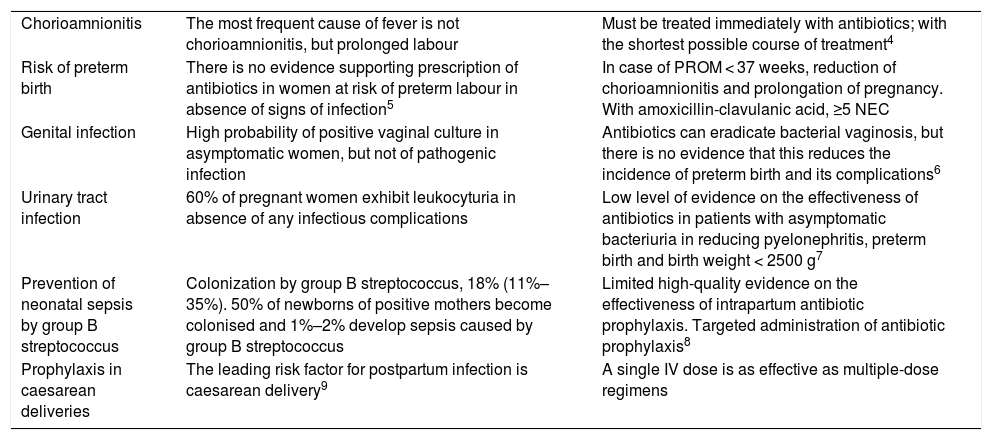
Table 1 presents the most frequent reasons for prescription of antibiotics in this period and the current evidence on these uses.4–9
Indications for antibiotic prescription during pregnancy. Indications and evidence.
| Chorioamnionitis | The most frequent cause of fever is not chorioamnionitis, but prolonged labour | Must be treated immediately with antibiotics; with the shortest possible course of treatment4 |
| Risk of preterm birth | There is no evidence supporting prescription of antibiotics in women at risk of preterm labour in absence of signs of infection5 | In case of PROM < 37 weeks, reduction of chorioamnionitis and prolongation of pregnancy. With amoxicillin-clavulanic acid, ≥5 NEC |
| Genital infection | High probability of positive vaginal culture in asymptomatic women, but not of pathogenic infection | Antibiotics can eradicate bacterial vaginosis, but there is no evidence that this reduces the incidence of preterm birth and its complications6 |
| Urinary tract infection | 60% of pregnant women exhibit leukocyturia in absence of any infectious complications | Low level of evidence on the effectiveness of antibiotics in patients with asymptomatic bacteriuria in reducing pyelonephritis, preterm birth and birth weight < 2500 g7 |
| Prevention of neonatal sepsis by group B streptococcus | Colonization by group B streptococcus, 18% (11%–35%). 50% of newborns of positive mothers become colonised and 1%–2% develop sepsis caused by group B streptococcus | Limited high-quality evidence on the effectiveness of intrapartum antibiotic prophylaxis. Targeted administration of antibiotic prophylaxis8 |
| Prophylaxis in caesarean deliveries | The leading risk factor for postpartum infection is caesarean delivery9 | A single IV dose is as effective as multiple-dose regimens |
We now proceed to summarise some conditions that demonstrate the negative impact of antibiotic use during pregnancy or intrapartum.
The disruption of the intestinal microbiota of the foetus and infant and its impact on the maturation of the microbiome in the child and adult are alarming, as the maternal and neonatal microbiomes influence the ontogeny of immune cells, in the offspring during gestation and in early postnatal life thereafter.10–14 The role of the perinatal microbiome in immunity and in the processes involved in normal neurodevelopment is complex, but it is known that alterations in the interactions between the microbiome and the nervous and immune systems in early life are associated with an increased risk of childhood atopy, asthma, allergy, obesity and neurologic disoders.12 A single course of antibiotics can change the bacterial flora with unwanted effects in the maternal and foeto-placental microbiomes that do not fully revert to baseline after treatment. Prenatal exposure to antibiotics in the second or third trimester is associated with an abnormal intestinal microbiome composition in the infant and an increased risk of childhood obesity: the risk of obesity increased by 84% in children exposed to antibiotics. Early postnatal development of the immune system is stimulated by the maternal microbiome during pregnancy. Interfering with this process may delay or even hinder the natural process of immune imprinting. Autoimmune and allergic diseases, including childhood asthma, may start in childhood and manifest later in life. A prospective study in a cohort of children whose mothers received antibiotherapy in the third trimester of pregnancy found an increased risk of asthma.
The use of intrapartum antibiotics in women in spontaneous preterm labour with intact membranes is not beneficial and has been associated with an increased risk of cerebral palsy and functional impairment in the offspring at age 7 years.15
Antibiotic prophylaxis for prevention of group B streptococcal disease is used in up to 40% of deliveries. Infants exposed to prophylaxis have been found to have decreased bacterial diversity, a decreased proportion of Actinobacteria, especially Bifidobacteriaceae, and a greater proportion of Proteobacteria in the intestinal microbiota compared to infants that were not exposed.10
In addition, the use of antibiotics during pregnancy is associated with an increased risk of hospital admission due to infection in the first 6 years of life (hazard ratio, 1.18; 95% confidence interval [CI], 1.17–1.19).16
Another potential complication of antibiotherapy is maternal anaphylaxis, which may cause the death of the mother and/or affect foetal oxygenation, which carries a risk of hypoxic-ischaemic encephalopathy and permanent central nervous system damage in the child.
Prenatal exposure to antibiotics has been associated with an increased risk of epilepsy in childhood. One study reported an incidence in children with prenatal antibiotic exposure of 117 per 100 000 person-years and a relative risk of 1.40 (95% CI, 1.22–1.61).17
There are still many unknowns as regards the adverse effects of cumulative exposure to antibiotics in the most vulnerable periods of human development. The Fetal Antibiotic EXposure (FAX) study currently underway analyses the association between intrauterine exposure to antibiotics and adverse outcomes in childhood, including body weight, allergic diseases and autism spectrum disorder. The initial cohort included 223 431 newborn infants in Southern California, of who 66% were exposed to antibiotics in utero. At 5 years, 80% of participants remained in the cohort and had made several medical visits throughout the followup,18 and we eagerly await the final results addressing key questions on the long-term sequelae of intrauterine antibiotic exposure.
Antibiotic overuse in the neonatal periodUnfortunately, antibiotics are also misused in neonatal clinical practice. The identification of sepsis, especially early-onset sepsis, is extremely inefficient. Despite the very low frequency of positive cultures in the NICU, antibiotics are the most utilised drugs in these setting, with high variability in usage between units.
A cross-sectional study in 326 845 newborns found that the percentage of exposure to antibiotics varied widely (ranging from 1.6% to 42.5%) and was not correlated to the presence of confirmed infection.19
Ideally, antibiotic use should be targeted with precision so that only infants with proven infection would receive antibiotics, and even then, only the narrowest-spectrum effective antibiotic.20
Suspected early-onset neonatal sepsis is one of the most common diagnosis in the NICU setting. The problem is that the suspicion of early-onset neonatal sepsis is confirmed in very rare cases (1%–3%). The assumption in many newborns that there is infection when there is not leads to overuse and misuse of antibiotics. Puopolo et al.21 have published an excellent description of the management of newborns with suspected or confirmed bacterial sepsis, in both preterm and term newborns (≥35 weeks’ gestation) (Table 2).
Considerations for the management of newborn infants with suspected early-onset neonatal sepsis.
| The estimation of the risk of infection and the decision to initiate antibiotherapy on account of suspected early-onset sepsis must be based on the clinical condition of the newborn |
| Do not use inflammatory markers in asymptomatic newborns |
| Consider the risks and benefits of empirical antibiotherapy |
| Preterm infants delivered by caesarean section due to a maternal non-infectious condition or placental insufficiency in absence of spontaneous labour, induction of labour or premature rupture of membranes are at very low risk of early-onset sepsis |
| Abnormal results of blood tests rarely justify prolonged empirical antibiotic use |
| Persistent haemodynamic instability is common in preterm newborns and not an indication in itself for prolonged empirical antibiotherapy |
Adapted from Puopolo et al.21.
It has been many years since physicians were first clearly warned that prolonged antibiotherapy for management of “culture-negative” sepsis is not good clinical practice22 and that irrational antibiotic use includes failing to use narrow-spectrum agents when appropriate.23 Yet recent reports show that antibiotics are widely used, with high variability between NICUs. For instance, in the Canadian Neonatal Network the overall proportion of newborns that received antibiotics was greater than 40%, although proportions ranged from 20% to 70%.2 The network of the Sociedad Iberoamericana de Neonatología (Iberoamerican Society of Neonatology, SIBEN), which includes 40 NICUs in 10 Latin American countries, reported proportions of administration of antibiotics for more than 3 days in newborns with negative blood cultures ranging from 10% to 92% and found an association between prolonged antibiotic use and mortality.
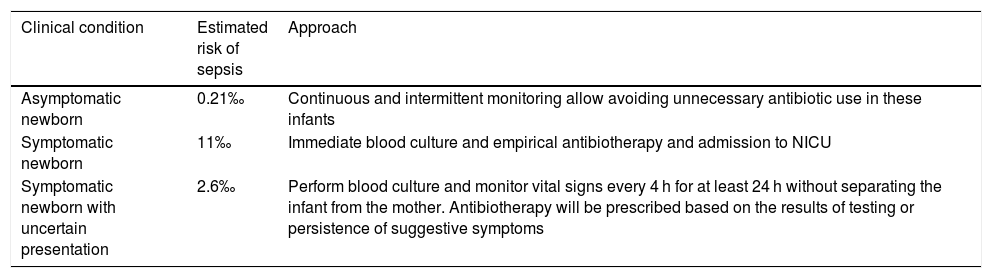
The clinical evaluation and risk assessment, based on continuous or intermittent serial monitoring, allows avoiding unnecessary use of antibiotics in these infants24,25 (Table 3).
Approach to management based on the risk of sepsis.
| Clinical condition | Estimated risk of sepsis | Approach |
|---|---|---|
| Asymptomatic newborn | 0.21‰ | Continuous and intermittent monitoring allow avoiding unnecessary antibiotic use in these infants |
| Symptomatic newborn | 11‰ | Immediate blood culture and empirical antibiotherapy and admission to NICU |
| Symptomatic newborn with uncertain presentation | 2.6‰ | Perform blood culture and monitor vital signs every 4 h for at least 24 h without separating the infant from the mother. Antibiotherapy will be prescribed based on the results of testing or persistence of suggestive symptoms |
Although the aim of this review is not to provide details on the deleterious impact of nonspecific inflammatory markers as regards the abuse of antibiotics in neonatal care worldwide, it is important to take into account how inaccurate they are. Procalcitonin, even at 12 h post birth, is not useful for clinical decision-making (sensitivity, 83%; specificity, 56%; positive predictive value, 32%; negative predictive value, 93%).26 A recent review and meta-analysis revealed the “waste” that has resulted from the use of C-reactive protein (CRP) for assessment of sepsis after 72 h post birth; evidence from 22 studies that included a total of 2255 newborns showed that the yield of CPR was very low (sensitivity, 0.62; specificity, 0.74), and therefore the results of this test, whether positive or negative, are of little value to modify the pretest probability of sepsis.27,28
Consequently, if antibiotherapy has been initiated and the blood cultures are negative and laboratory tests are normal, antibiotherapy must be discontinued.
The reported incidence of early-onset sepsis in newborns of mothers with clinical chorioamnionitis is low, ranging from 1‰ to 4‰ to 7‰. Therefore, there is no need to treat all asymptomatic newborns with a history of chorioamnionitis.29–31 If antibiotherapy was initiated in all asymptomatic newborns of mothers with chorioamnionitis, there would be hundreds (maybe thousands) of admissions per single case of neonatal sepsis confirmed by culture. This reflects the extremely high number needed to treat to identify a newborn with confirmed early-onset sepsis, as the number needed to treat is high even in symptomatic infants born to mothers with chorioamnionitis, of 23 or greater.
It is therefore clear that the routine and immediate prescription of antibiotherapy is not justified in most situations. The first 24 h of life are critical. How can we deny newborns the benefits of repeated and detailed clinical evaluations? With this practice we avoid interfering with mother-child bonding and we can quickly establish the need for intervention.32
In short, routine and immediate use of antibiotherapy in neonatal care is not justified in most situations. There is a pressing need for a more rational use of antibiotics in newborns, discouraging routine use of empirical antibiotherapy when sepsis is only a remote possibility.
It is our duty to strive to counteract deeply entrenched attitudes based on tradition and support changes in clinical practice to ensure that improvements in neonatal health become solidly established in all NICUs worldwide.
Negative short- and long-term impact of antibiotic use in the neonatal periodProlonged exposure to antibiotics in the neonatal period is associated with significant morbidity such as systemic candidiasis, necrotising enterocolitis (NEC), late-onset neonatal sepsis, bronchopulmonary dysplasia, retinopathy of prematurity, emergence of multidrug-resistant microorganisms, alterations in the intestinal microbiome for many years and increased mortality. Many articles on the subject have been published in recent years.33 Several of these sources report an increased mortality. The SIBEN network has also found an association between overuse of prolonged antibiotherapy and mortality in infants with negative cultures.
Antibiotherapy in the first and second years of life is associated with a 10%–15% increase in the risk of childhood obesity, and other authors have reported an increased risk of childhood asthma and other allergic diseases, coeliac disease, Crohn disease (7-fold risk), juvenile idiopathic arthritis and possibly intestinal cancer.34
Antimicrobial resistanceThe introduction of antibiotics was one of the greatest breakthroughs in medicine in the 20th century. However, their misuse has brought on a rapid increase in antimicrobial resistance so that some infections are now very difficult to treat or untreatable. According to a report of the United Nations and another of the United Kingdom, by 2050, 10 million people will die across the world each year due to drug-resistant infections unless doctors worldwide make significant changes to their antibiotic prescription practices.35
What can be done to reduce antibiotic misuse in neonatal care?Studies assessing the impact of antimicrobial stewardship and auditing programmes in the NICU setting have reached different conclusions regarding their effectiveness in improving antibiotic prescription practices.23,36–41
The antibiotics used most frequently in neonatal care are ampicillin and gentamycin. Initiation of both is recommended in case of suspected early-onset sepsis, with discontinuation at 48–72 h in case of negative cultures. However, a recent study, as an example, shows that this is not implemented consistently and described not only a mean duration of antibiotherapy of 10.8 ± 7.3 days, but also the wholly inappropriate use of carbapenem and vancomycin for initial treatment. When it comes antimicrobial stewardship, consideration of the clinical context, the tools used for diagnosis, the choice of drug and the duration of therapy are key factors to address in the auditing and real-time feedback processes of quality improvement programmes. Multidisciplinary teams can develop and implement effective interventions to decrease antibiotic use in the NICU setting. The main goals are to decrease the frequency of initiation of antibiotherapy in newborns and the days of treatment per 1000 patient-days, maintaining or even improving safety outcomes. An exhaustive evaluation of antibiotic usage ought to be conducted in each NICU to identify and implement interventions adapted to specific facilities. For instance, a policy of automatic discontinuation proved effective in reducing the mean number of days of antibiotherapy per patient and the mean number of excessive days of antibiotherapy. Such automatic discontinuation authorises the programme team to suspend antibiotherapy in case of inappropriate antimicrobial spectrum or excessive duration of treatment. In addition, the newborn sepsis calculator to estimate the risk of early-onset sepsis is an effective tool for optimising antibiotic usage in neonatal care and its use has been associated with a decrease in the use of empirical antibiotherapy.40,41 The application of this tool achieved significant decreases in the diagnostic tests used in relation to suspected sepsis and in NICU lengths of stay. This practice was also associated with a mean decrease in health care costs of 207Є per admitted infant.41 Daily auditing and feedback led to an overall decrease in antibiotic use of 14% in extremely preterm newborns, but there was no evidence of a positive impact of treatment in the subset of infants in who antibiotherapy continued despite negative culture results or in the duration of antibiotherapy.
Last of all, several reviews and meta-analysis have evinced the benefits of antimicrobial stewardship programmes in improving antibiotic prescription practices in hospitalised patients.42,43
Economic impact of antibiotic overuseReducing antibiotic misuse and overuse achieves decreases in direct and indirect costs. Switching initial treatment from wide-spectrum antibiotics, which are more costly, to other, more inexpensive ones, reducing the number of patients in who antibiotherapy is initiated unnecessarily, timely discontinuation to avoid prolonged antibiotherapy combined with subsequent decrease in performance of blood cultures and other laboratory tests and the reduction in the incidence of drug-resistant infections achieve significant savings by preventing unnecessary health care expenditures. In addition, these improvements reduce the incidence of adverse drug events and the length of stay.
An analysis of antibiotic expenditures in clinical practice in the United States revealed a global expenditure in antibiotics of about $9.7 billion: $6.2 billion at the outpatient level and $3.5 billion at the inpatient level, with the total expenditure for the 2010–2015 6-year period estimated at $56 billion.44
If we take the reported reduction in costs of Є207 per hospitalised newborn41 and extrapolate it to 3 or 4 NICUs that cumulatively admit 1000 patients per year, the savings would amount to 207 000Є (approximately $230 000) in these units alone.
ConclusionsIt is estimated that without implementation of restrictions and local stewardship programmes in high-, medium- and low-income countries, the global antibiotic use will have tripled by 2030. These projections estimate, absent any changes to current clinical practices, an increase by more than 200% of the more than 42 000 daily defined doses estimated to have been administered in 2015. What can perinatal care teams do to prevent this?
One important strategy related to antibiotic use in perinatal care is the protection of mother and child from infection. It is unquestionable that this improves patient outcomes. However, this protection should not come at the expense of risking harm to uninfected mothers and infants. Establishing an appropriate “equilibrium” in antibiotic usage would allow standardised evaluation of the risk and benefits involved in antibiotherapy and reduce antibiotic overuse.
The indication for medical prescription is defined as “the careful and responsible management of something entrusted to one’s care”. The use of antimicrobials is one of the three “pillars” of an integrated approach to strengthen health care systems. The Centers for Disease Control and Prevention (CDC) has made efforts to achieve adequate improvements in antibiotic usage in inpatient and outpatient settings45 and described the key elements to consider in antibiotic use, acknowledging that there is no single or universal approach appropriate for all settings. In some cases, good practice involves early initiation of wide-spectrum antibiotics, but in others good practice involves avoiding or discontinuing antibiotherapy. The need to improve antibiotic overuse in perinatal care in developed and low- and middle-income countries has never been more urgent. It is our responsibility to pursue this goal in our daily activity in each obstetrics, maternity and neonatal intensive care unit.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cardetti M, Rodríguez S, Sola A. Uso (y abuso) de antibióticos en la medicina perinatal. An Pediatr (Barc). 2020;93:207.