The management of urinary tract infection (UTI) in infants and children has changed significantly over the past few decades based on scientific evidence that questioned the efficacy of strategies used to prevent kidney injury and subsequent progression to chronic kidney disease, which is very unlikely in most paediatric cases. However, there is still substantial heterogeneity in its management and uncertainty regarding the diagnosis, indication of imaging tests, treatment or follow-up in these patients. The Spanish clinical practice guideline has been updated through the review of the literature published since 2009 and a rigorous evaluation of current clinical practice aspects, taking into account the evidence on the benefits of each intervention in addition to its risks and drawbacks to attempt to provide more precise recommendations.
En las últimas décadas, se han producido profundos cambios en el manejo de la infección urinaria en pediatría, a partir de estudios que cuestionaban la eficacia de las estrategias para prevenir el daño renal y la evolución posterior a enfermedad renal crónica, muy poco probable en la mayoría de casos. No obstante, todavía existe una gran variabilidad clínica en el manejo e incertidumbre en cuestiones relativas al diagnóstico, la indicación de una exploración radiológica, el tratamiento o el seguimiento de estos niños. La actualización de la guía de práctica clínica española ha examinado en la literatura publicada desde el año 2009 y de modo riguroso aspectos de la práctica actual, considerando la evidencia de los beneficios de cada intervención, junto con los riesgos asociados y desventajas, para intentar delimitar las pautas de actuación más precisas.
Urinary tract infections (UTIs) are among the most frequent infections in children and, while the outcome is good in a large proportion of cases, their occurrence may result in a progressive loss of renal function, either in association with renal dysplasia or recurrent episodes of pyelonephritis. Thus, clinical algorithms need to be developed to identify this small but important subset of patients.1 The customary management of these children includes imaging tests, chemoprophylaxis and long-term follow-up, which are inconvenient to patients and families and entail an excessive use of health care resources. The lack of scientific consensus on the management of UTIs motivated the development of the first version of the Spanish clinical practice guideline (CPG) in 2011, with a strong determination to establish specific guidelines in order to reduce the substantial variability in clinical practice.2
The effort yielded a CPG of high methodological quality,3 in spite of which the heterogeneity in the management of this condition in Spain still persists. Since its publication, multiple consensus documents and updates of other guidelines have been published taking into account the findings of new studies that clarified some of the most controversial aspects.1,4–8 In consequence, the working group considered that it would be appropriate to update the Spanish CPG on those subjects on which there has been the largest number of relevant research publications to address aspects that continue to generate uncertainty, with the purpose of providing a rigorous evidence-based tool to guide the diagnosis, treatment and follow-up of UTI (Appendix A, supplementary data 2).
The target population for this guideline are all children at risk of having a UTI aged 1 month to 18 years. The guideline also addresses the management of paediatric patients who require short-term bladder catheterization for diagnostic or therapeutic purposes. The main aspects addressed in this update are summarised in the present document, although we recommend consultation of the full text9 to read the scientific evidence that supports the recommendations.
MethodsThe methodology used to develop the CPG is explained in the Methodological Manual for the Development of Clinical Practice Guidelines of the Spanish National Health System,10 and the working group that developed the update included 14 medical practitioners active in different health care settings (primary care and hospital) and fields (paediatrics, surgeryand paediatric nursing, infectious disease and nephrology). The working group also included other professionals who provided methodological support and contributed to the literature search and review. We also consulted potential users of the information developed for patients to review these contents (Appendix A, supplementary data 3).
To formulate the revised clinical questions, we continued to use the PICO (patient-intervention-comparison-outcome) approach. We performed a literature search for original articles in the PubMed/MEDLINE, Embase and Cochrane Library databases, restricting the search to articles published between January 1, 2009 and April 2021. Additional sources were obtained through the setting of alerts for newer publications, backward reference searching and manual searches conducted by working group members. The search strategies combined controlled and natural language terms, focusing on the most appropriate type of studies for each question, and limited to publications in English, French, Italian, Portuguese and Spanish.9 The first screening of the articles was based on the titles and abstracts. The second involved reading of the full text and assessment of the risk of bias applying the critical reading tool of the Department for Assessment of Health Care Technology of the Basque Country.11 We graded the quality of the evidence and developed recommendations based on the methodology proposed by the Scottish Intercollegiate Guidelines Network (SIGN) (Appendix A, supplementary data 4 and 5).
After the critical reading of the evidence, the group proceeded to the development of the recommendations following the formal evaluation model with plenary meetings. In addition to the amount and quality of the evidence, the group considered the consistency of the findings and their relevance in terms of their application to the Spanish health care system or their clinical impact. In the case of clinical questions for which the evidence was scarce, of low quality (levels of evidence 1- or 2-) or inconsistent, the recommendations were established by consensus. For questions for which we considered evidence from meta-analyses for any of the outcomes of interest, we took into account the clinical and statistical heterogeneity. After developing the initial draft, the text underwent external review. The external reviewers were proposed by members of the working group or by the scientific societies that they represented.
Summary of the recommendationsDefinition and classificationFrom a conceptual perspective, the construct of an ITU involves the growth of microbes in the urinary tract combined with compatible symptoms, to be distinguished from asymptomatic bacteriuria, which does not cause symptoms and does not require treatment. From a practical standpoint, symptomatic UTI can be classified as UTI involving the renal parenchyma (acute pyelonephritis [APN]) and not involving the kidneys (cystitis). Although in clinical practice the term febrile UTI is frequently used interchangeably with APN, fever in the context of a UTI is not always associated with renal involvement.2
On the other hand, a UTI is considered recurrent if a patient experiences 2 or more episodes of APN, 1 episode of APN and 1 or more episodes of cystitis or 3 or more episodes of cystitis in a single year. Lastly, a UTI is considered atypical if it is associated with sepsis, an abdominal or vesical mass or elevation of serum creatinine, if there is no response to treatment within 48–72 hours or if it is caused by an organism other than extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli.2
Clinical diagnosisThe signs and symptoms of UTI in children are highly nonspecific (Table 1). In infants and young children without bladder continence, high fever without a source in combination with certain additional manifestations is associated with an increased probability of UTI,12,13 while in older children with bladder continence, urinary symptoms are more prominent.14 The combination of several signs and symptoms increases the diagnostic yield, but testing is still required to confirm the UTI.
Signs and symptoms of UTI in infants and children with UTI.2
| Age group | Signs and symptoms | |||
|---|---|---|---|---|
| Most common | ↔ | Least common | ||
| Infants younger than 3 months | FeverVomitingLethargyIrritability | Poor feedingFailure to thrive | Abdominal or suprapubic painJaundiceHaematuriaFoul-smelling and/or cloudy urine | |
| Infants and children, 3 months or older | Preverbal | Fever | Abdominal or suprapubic painLumbar painVomitingPoor feeding | LethargyIrritabilityHaematuriaFoul-smelling and/or cloudy urineFailure to thrive |
| Verbal | FrequencyDysuria | Changes to continenceAbdominal or suprapubic painLumbar pain | FeverMalaiseVomitingHaematuriaFoul-smelling and/or cloudy urine | |
Recommendations
- -
Clinical suspicion of UTI in paediatric patients based on signs and symptoms requires laboratory confirmation due to the low discriminant power of these features. Grade of recommendation: A.
- -
In infants and children aged less than 24 months with fever without source, we recommend urine testing to rule out UTI, especially if the fever is high (>39 °C) and prolonged (>48 h). Grade of recommendation: A.
- -
In children aged more than 24 months presenting with abdominal or lumbar pain in the context of fever, dysuria, urinary frequency or both or development of incontinence, confirmation of UTI through a urine test is recommended. Grade of recommendation: A.
The urine collection method is a crucial element for the reliable diagnosis of UTI. The method must be selected taking into account the clinical condition of the patient, the level of bladder control and cooperation and the resources of the health care setting (invasive techniques are not generally used at the primary care level) (Table 2). To decrease the likelihood of contamination using non-invasive methods,15 we suggest using bladder stimulation techniques for collection of spontaneous clean urine catch samples in young infants, which have been found to achieve promising results with acceptable values in validity indicators.16,17 Independently of the collection method, the sample should be processed within 4 h of collection and, whenever this is not possible, refrigerated immediately for a maximum of 24 h.2
Urine sample collection methods. Advantages, drawbacks and indications.4
| Positive urine culture | Advantages | Drawbacks | Indication | |
|---|---|---|---|---|
| Clean catch | ≥50,000–100,000 CFU/mL of a pathogena | - Acceptable validity indicators- Non-invasive- Simple | - Risk of contamination depending on hygiene and sterile technique | All children with bladder continence* |
| Perineal bag | Not recommended for urine culture samples | - Non-invasive- Simple | - High false positive rate (>50%–60%)- Needs confirmation if results are positive | Useful for initial screening in non-urgent cases in children without bladder continence |
| Catheter | ≥10,000 CFU/mL of a pathogena | High sensitivity and specificity | - Invasive- Risk of urethral trauma- Some risk of contamination(10%–26%) | Confirmation method in children without bladder continence and initial method in urgent cases ** |
| Suprapubic aspiration | Any amount of growth | Gold standard | - Invasive- Variable success (30%–70%)- Ideally, guided by ultrasound | Confirmation method in children without bladder continence and initial method in urgent cases ** |
CFU, colony-forming units; UTI, urinary tract infection.
Recommendations
- -
In the paediatric population with bladder continence, collection of midstream clean catch urine samples is recommended. Grade of recommendation: B.
- -
In patients currently incontinent who require immediate diagnosis and/or treatment, we recommend selection of a method that minimises the risk of contamination (midstream/spontaneous urine catch, urinary catheter or suprapubic aspiration samples). Grade of recommendation: C.
- -
In the case of spontaneous urine collection, we recommend the use of bladder stimulation techniques, especially in infants aged less than 6 months. Grade of recommendation: A
- -
In paediatric patients without bladder continence who do not require urgent diagnosis or treatment, non-invasive urine collection methods can be used if performed correctly. Collection of spontaneous urine over the perineal bag is preferred. Grade of recommendation: C.
- -
If urine testing yields abnormal results in samples collected with a method that carries a high risk of contamination (perineal bag or sterile pad), confirmation of the results in a different sample collected with a technique that minimises the risk of contamination is recommended. Grade of recommendation: D
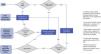
Traditional tests are based on the use of dipstick urinalysis or microscopic urinalysis, manual or automated, and Gram staining. The values of some laboratory tests in urine are associated with an increased or decreased probability of a UTI diagnosis in cases with clinical suspicion and can guide decisions regarding the initiation of antibiotherapy (Fig. 1).13 Recent studies have demonstrated the significance of the severity of leukocyturia as a finding that increases the likelihood of a UTI, which facilitates rapid presumptive diagnosis in young children.13,18 However, it is important to take into account that pathogens like Enterococcus spp, Klebsiella spp or Pseudomonas spp can cause UTIs that manifest with milder inflammatory signs early in the infection.19 In any case, confirmation of UTI by urine culture, the interpretation of which will be adapted to the sample collection method, is recommended (Table 2).
Recommendations
- -
In patients aged less than 2 years or without bladder continence with suspected UTI based on clinical manifestations, microscopic urinalysis (or, if not available, dipstick urinalysis) is recommended, and, if the results are abnormal, urine culture should be collected as well as a Gram staining in infants younger than 3 months. Grade of recommendation: B
- -
In patients aged less than 2 years or without bladder continence with suspected UTI, initiation of empiric antibiotherapy is recommended after collection of the urine sample for culture if there is bacteriuria, a positive nitrite test or a significantly high white blood cell (WBC) count (≥2+ in dipstick or ≥20 WBC/hpf in microscopy) in a valid urine sample. Grade of recommendation: B.
- -
In patients aged more than 2 years in whom UTI is strongly suspected (specific symptoms, positive nitrites, bacteriuria or high WBC count), initiation of empiric antibiotherapy is recommended after collection of the urine sample for culture. Grade of recommendation: B.
- -
If nitrites or a high WBC count are not detected in the urinalysis and the symptoms are nonspecific, urine culture and empiric antibiotherapy are not recommended at the outset. Grade of recommendation: B.
- -
Negative flow cytometry or automated microscopic urinalysis results are not sufficient grounds to decide against collecting a urine sample and performing urine culture. Grade of recommendation: B.
Localisation of a UTI is important from a therapeutic and prognostic perspective, and while fever may be a warning sign of APN, the assessment of urinary tract involvement with a dimercaptosuccinic acid (DMSA) scintigraphy scan is considered the gold standard of diagnosis, as the signs and symptoms are nonspecific.20 In real-world clinical practice, the elevation of acute phase reactants is the most accurate biomarker,21 although its actual usefulness may be limited due to the high pre-test probability of selected cases.
Recommendations
- -
Acute pyelonephritis should be suspected in patients with elevated levels of C-reactive protein (CRP) and/or procalcitonin (PCT), especially the latter. Grade of recommendation: C.
- -
The probability of APN should be considered low if fever, abdominal pain and general malaise are absent and acute phase reactant values are normal or only mildly elevated (CPR <20 mg/L, PCT <0.5 ng/mL, erythrocyte sedimentation rate [ESR] <10 mm/hour and/or serum interleukin-6 [IL-6] <4 pg/mL). Grade of recommendation: B.
- -
Acute pyelonephritis should be suspected in patients with decreased renal concentrating capacity. Grade of recommendation: √.
- -
Consider performance of blood tests (complete blood count, basic chemistry panel and urea, creatinine, electrolytes and acute phase reactants) in patients who meet the criteria for hospital admission, with an unfavourable course of disease and with risk factors for renal damage or unfavourable outcomes, such as recurrent UTI, urinary system abnormalities in the prenatal ultrasound, high fever or a high probability of an atypical pathogen. Grade of recommendation: √.
Used to detect abnormalities that could be associated with the development of a new UTI as well as any renal damage to ensure appropriate follow-up. The algorithms proposed in different guidelines vary widely, ranging from thorough imaging evaluations including cystourethrography and DMSA scintigraphy22 to more basic screening starting with a renal ultrasound.1,4,6–8 Although there is sufficient evidence on the validity of imaging tests, there is not as much evidence on their yield in clinical practice. There is also controversy due to the uncertainty whether the active search for vesicoureteral reflux (VUR) and its subsequent correction actually change patient outcomes.23 In any case, the association between urinary tract malformations and permanent renal scarring is well established,24–26 so the need for early screening for VUR or screening in patients with recurrent UTI continue to be a subject of debate. Along the same lines, estimating the negative predictive value of the absence of abnormalities in the prenatal ultrasound at the local level is also necessary. Thus, most recommendations are based on expert consensus or low-grade evidence, and a balance must be sought between the need to conduct an adequate evaluation and the effort to minimise the risks and costs to the patient and to the health care system27,28 (Fig. 2).
Acute managementIt usually starts with empiric antibiotherapy, which requires up-to-date knowledge of local antimicrobial resistance patterns, taking into account that E coli is the most frequent aetiological agent (60%–80% of cases). Antimicrobials with a local prevalence of resistance greater than 10%–15% should not be considered for empiric antibiotherapy.2,29 Previous exposure to antibiotics, a history of hospitalization or the presence of kidney and urinary tract anomalies increase the likelihood of other aetiological agents, such as Proteus mirabilis, Klebsiella spp or Pseudomonas aeruginosa.Enterococcus faecalis should also be considered in young children or patients with significant uropathy.9,30
Overall and in the general population, E coli in Spain has adequate sensitivity to second- and third-generation cephalosporins, aminoglycosides, fosfomycin and nitrofurantoin. However, there is a high prevalence of resistance to amoxicillin or ampicillin (>60%) and cotrimoxazole (20%–40%). On the other hand, there is an increasing trend in the resistance of E coli to first-generation cephalosporins and amoxicillin-clavulanic acid, already exceeding 10%–15% in parts of Spain. There has also been an increase in fluoroquinolone resistance in E coli in the general population, although the few paediatric case series that analysed resistance to these antimicrobials found very small percentages of resistance. The susceptibility profile of P mirabilis is similar to that of E coli, although some series have found a lower susceptibility to fosfomycin. Klebsiella pneumoniae is naturally resistant to ampicillin and remains highly susceptible to other antimicrobials commonly active against it, although the emergence of BLEE-producing strains may explain the significant decrease in susceptibility to cephalosporins observed in some series. Pseudomonas aeruginosa continues to exhibit adequate susceptibility to carbapenems, piperacillin-tazobactam, ceftazidime, tobramycin and amikacin, while the susceptibility to ciprofloxacin and gentamicin has decreased slightly.
Thus, given that no antibiotic guarantees 100% coverage, a combination may be required in high-risk UTI cases. The combination should include ampicillin to cover for Enterococcus spp, and specific antibiotics against P aeruginosa should be added if coverage for this species is required. Last of all, although infection by multidrug-resistant organisms is rare in children, carbapenems should be added to cover against these pathogens9 (Table 3).
Recommendations for choice and duration of empiric antibiotherapy.9
| Choice initial of antibiotica,b | ||
|---|---|---|
| Treatment | Antibiotic | Course length |
| Febrile UTI meeting criteria for hospital admission (IV route)c,d | Cefotaxime/CeftriaxoneGentamicin/Tobramycin*OthereAmoxicillin-clavulanic acid (5:1)eCefuroxime axetil | 7−10 daysg |
| Febrile UTI not meeting criteria for hospital admission (oral route) | CefiximaeAmoxicillin-clavulanic acid (4:1)eCefuroxime axetil | 7−10 days |
| Nonfebrile UTI (symptoms suggestive of lower urinary tract infection/cystitis) | Cefuroxime axetilf NitrofurantoinFosfomycin (age >12 years)eAmoxicillin-clavulanic acid (4:1)eCotrimoxazole | 3−4 daysh |
UTI, urinary tract infection.
In patients with suspected obstructive urinary tract disease and infants under 3 months, add ampicillin to the empiric antibiotherapy regimen if infection by Enterococcus spp is considered possible.
Once susceptibility results become available, antibiotic treatment, whether IV or oral, should be de-escalated accordingly, and select the antibiotic seeking the best penetration on the renal parenchyma, lowest toxicity, best possible tolerance and the lowest possible spectrum.
Cefotaxime is the first-line option in patients with a history of renal scarring and should be given at a high dose in the case of sepsis or meningitis.
When treatment with aminoglycosides is required, use once-daily dosing and adjust the dose as needed based on therapeutic drug monitoring.
Other third-generation cephalosporins, such as ceftazidime, and other antibiotics, such as amikacin, carbapenems, and quinolones should be reserved for special circumstances.
In Spain, its prolonged use for prophylaxis is not authorised due to the potential pulmonary and hepatic risks.
The antibiotherapy approach has not changed in recent years, and oral antibiotherapy is preferred, even for febrile UTI cases,4,5,31 although a delay in initiation greater than 48 h increases the risk of permanent renal scarring.32 The need of hospital admission is determined based on the specific circumstances, clinical presentation and course of disease and risk factors, although outpatient parenteral antibiotic therapy can be considered as an alternative.1,2,4,29 A clinical evaluation of the patient every 48 h is recommended, and, if antibiotherapy started via the intravenous route, stepping down to oral treatment as soon as the condition of the patient allows it, along with de-escalation based on susceptibility testing results9 (Appendix A, supplementary data 6).
When it comes to the use of adjuvant treatment to prevent renal scarring in children with pyelonephritis,33,34 the addition of dexamethasone to the customary antibiotic has not been found to reduce the risk of renal scarring (relative risk, 0.56; 95% confidence interval, 0.3–1.07),9 so its routine use is not recommended (Fig. 3).
Recommendations
- -
In the case of a well-founded suspicion of febrile UTI, early initiation of antibiotherapy is recommended. Grade of recommendation: C.
- -
In children with febrile UTI without known obstructive uropathy and without symptoms of severe infection, oral antibiotherapy is recommended. Grade of recommendation: A.
- -
In children with VUR grade III to V, antibiotherapy should be initiated via the intravenous route. Grade of recommendation: B.
- -
In children aged less than 2–3 months or with suspected uropathy, signs of sepsis, refractory vomiting or dehydration, antibiotherapy should be initiated via the intravenous route. Grade of recommendation: √.
The general measures used to reduce the risk of recurrence include adequate fluid intake, healthy voiding habits and management of constipation.1,2,4,29 Prolonged antibiotic prophylaxis does not protect from the development of new UTIs in children without associated malformations and promotes the development of antimicrobial resistance.12,23 On the other hand, in children with VUR, prolonged antibiotic prophylaxis seems to offer protection against new UTIs based on studies with a low risk of bias9 (Figs. 4 and 5), and to be particularly effective in patients with bladder and bowel dysfunction, although it does not have an impact on the development of additional renal scarring35,36 It has also been found to be effective in preventing UTI in patients with prenatal dilatation associated with VUR, especially in girls, although it also did not prevent new renal scarring and was associated with an increase in antimicrobial resistance37 (Fig. 6). Alternative preventive treatments that can be considered to avoid the promotion of antimicrobial resistance include cranberry extract38 and probiotics,39,40 although the current evidence is limited to studies in low-risk patients and is less consistent for the latter.
Recommendations
- -
Local antimicrobial resistance patterns should be taken into account when considering prophylaxis, and selection of the agents with the narrowest-possible spectrum is recommended, with administration of one fourth to one third of the dose at night. Grade of recommendation: √.
- -
In patients with recurrent UTI and no structural abnormalities or with low-grade VUR, prophylaxis with cranberry extract should be contemplated. Grade of recommendation: B.
Follow-up of patients after a UTI is warranted due to the potential development of changes in the urinary system or renal scarring and the high probability of recurrence, and caregivers should be educated about the symptoms suggestive of a UTI and the need of early treatment.2,4,29 Routine urinalysis or urine culture is not recommended during antibiotic treatment if there is an adequate response or during follow-up after a UTI in the absence of symptoms, independently of the presence of structural abnormalities.2,4,29
Referral to a specialist is recommended if renal scarring is detected or in cases in which there is a high risk of renal scarring that requires assessment for confirmation: patients with structural or functional urinary system abnormalities, atypical or recurrent UTI or any other risk factors1,2,4,29 (Appendix A, supplementary data 7).
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank César Loris Pablo, clinical coordinator of the first Spanish clinical practice guideline for urinary tract infection in the paediatric population, and all authors, collaborators and reviewers of that foundational document.
We thank Montserrat Antón Gamero (Paediatric Nephrology, Hospital Universitario Reina Sofía, Córdoba), José Cristóbal Buñuel Álvarez (Paediatrics, Centro de Salud de Santa Isabel, Zaragoza), Daniel Cabezalí Barbancho (Paediatric Urology, Hospital Doce de Octubre, Madrid), Mar Espino Hernández (Paediatric Nephrology, Hospital Doce de Octubre, Madrid), Javier González De Dios (Paediatrics, Hospital General Universitario de Alicante), Roi Piñeiro Pérez (Paediatric Nephrology and Infectious Diseases, Hospital Universitario General de Villalba, Madrid), Cinta Sangüesa Nebot (Paediatric Radiology, Hospital Universitari i Politècnic la Fe, Valencia) and Roberto Velasco Zúñiga (Paediatric Emergency Department, Hospital Universitari Parc Tauli, Sabadell) for their participation as external reviewers of the updated Clinical Practice Guideline for Urinary Tract Infection in Infants and Children.
Also, María Esther García Pomar (Guía Salud, Instituto Aragonés de Ciencias de la Salud, Zaragoza) for her collaboration on this update.
We also express our gratitude to the Spanish Association of Paediatric Nephrology for its contribution to the publication, printing and working group meeting expenses. We must not forget the other scientific societies involved in the development of this guideline, represented by the members of the working group and the external reviewers: Spanish Association of Paediatrics, Spanish Association of Primary Care Paediatrics, Spanish Society os Paediatric Surgery, Spanish Association of Paediatric Nursing, Spanish Society of Paediatric Infectious Diseases, Spanish Society of Paediatric Radiology and Spanish Society of Paediatric Emergency.
In memory of Dr Luis Miguel Rodríguez Fernández, an honourable role model in life and in the medical profession.
Members of the Working Group for the Update of the Clinical Practice Guideline for Urinary Tract Infection in Infants and Children.
Joaquín Escribano Subías (ORCID 0000-0002-5041-459X). Paediatric Nephrology, Hospital Universitario Sant Joan Reus, Reus.
Gloria María Fraga Rodríguez (ORCID 0000-0002-7682-1396). Paediatric Nephrology, Hospital Universitario de la Santa Creu i Sant Pau, Institut de Recerca Sant Pau (IR Sant Pau), Universitat Autònoma de Barcelona.
César Joaquín García Vera (ORCID 0000-0003-3017-969X). Paediatrics, Centro de Salud Sagasta-Ruiseñores, Zaragoza.
Andrés Gómez Fraile (ORCID 0000-0003-0739-9897). Paediatric Surgery, Hospital Doce de Octubre, Madrid.
Juan David González Rodríguez (ORCID 0000-0002-8863-2007). Paediatric Nephrology, Hospital General Universitario Santa Lucía, Cartagena.
Leire Madariaga Domínguez (ORCID 0000-0002-4032-9842). Paediatric Nephrology, Hospital Universitario Cruces, Universidad del País Vasco, IIS Biobizkaia, Barakaldo.
Juan Ignacio Martín Sánchez (ORCID 0000-0001-8829-7985). Preventive Medicine and Public Health, Instituto Aragonés de Ciencias de la Salud, Zaragoza.
José María Mengual Gil (ORCID 0000-0002-0521-6316). Paediatrics, Centro de Salud Delicias Sur, Zaragoza.
Carlos Ochoa Sangrador (ORCID 0000-0002-7670-3699). Paediatrics, Hospital Virgen de la Concha, Zamora.
Gustavo Padilla Aragües (ORCID 0009-0008-2278-800X). Paediatric Intensive Care Nursing, Hospital Universitario Materno Infantil Miguel Servet, Zaragoza.
Ester Parada Ricart (ORCID 0000-0002-1696-036X). Paediatric Nephrology, Hospital Universitario Joan XXIII, Tarragona.
Neus Rius Gordillo (ORCID 0000-0001-7562-078X). Paediatric Infectious Diseases, Hospital Universitario Sant Joan Reus, Reus.
Yolanda Romero Salas (ORCID 0000-0003-2630-3467). Paediatric Nephrology, Hospital Universitario Materno Infantil Miguel Servet, Zaragoza.
Montserrat Salas Valero (ORCID 0000-0001-6261-3724). Documentation and Information Systems, Instituto Aragonés de Ciencias de la Salud, Zaragoza.
Agustín Serrano Durbá (ORCID 0000-0002-3158-8914). Urology, Hospital Universitario y Politécnico La Fe, Valencia.
Blanca Valenciano Fuentes. Paediatric Nephrology, Complejo Hospitalario Universitario Insular Materno Infantil, Las Palmas de Gran Canaria.