Recently, we reviewed the experience in our hospital in the management of acquired aplastic anaemia (AAA) and refractory cytopenia in childhood (RCC), in accordance with the recommendations and studies associated with the a European Society for Blood and Marrow Transplantation (EBMT).1,2 Acquired aplastic anaemia is the most common form of acquired bone marrow failure, with an estimated incidence of 1.5 cases per 2 million individuals per year in Europe.1 Refractory cytopenia in childhood is the most common myelodysplastic syndrome in children. It is recommended that these patients receive the same treatment established for AAA, based on haematopoietic stem cell transplantation (HSCT) or immunosuppressive therapy (IST).3 The aim of our study was to retrospectively assess the response rate, event-free survival and overall survival of patients with AAA and RCC that underwent HCST or IST.
The study included 48 patients with a diagnosis of AAA or RCC managed at the Hospital Infantil Universitario Niño Jesús between 2001 and 2020. Two patients (4.08%) did not received treatment, one due to spontaneous recovery and the other due to paroxysmal nocturnal haemoglobinuria (PNH) clone expansion, which did not require treatment until the patient was transferred to another centre. We defined complete remission (CR) as a neutrophil count of 1.5 × 109/L or greater, a platelet count of 150 × 109/L or greater and a haemoglobin concentration of 10.5–13 g/dL.1 We defined partial remission (PR) as the achievement of transfusion independence. The events taken into account in the analysis of event-free survival (EFS) were relapse and death. We used Kaplan-Meier survival analysis to generate cumulative incidence curves and compared the results by means of the log-rank test.
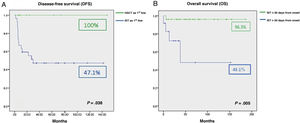
We found an overall survival (OS) in the case series of 90.6%. The 9 patients (18.75%) that underwent HLA-matched related donor HSCT achieved CR without requiring further treatment. In the remaining 37 patients (77.08%), IST was used for first-line treatment with a response rate of 73% (18/37 CR and 9/37 PR). Comparing the outcomes of initial treatment, we found that the OS with HSCT was 100% compared to 85% with IST as first-line treatment (P = .321). The comparison of HSCT versus IST for first-line treatment did find a significant difference when it came to EFS: 100% with HSCT as first-line treatment compared to 47.1% with IST (P = .038) (Fig. 1A). The response rate in the 13 patients that required additional courses of IST was 61.5% (4/13 CR and 4/13 PR). The EFS with HSCT as second-line treatment was 62.5% compared to 50% with IST as second-line treatment. Of the 5 patients that did not respond to the second course of IST, 3 received transplants from alternative donors, which achieved an increase of OS with IST as second-line treatment to 83.9%. The median time elapsed from diagnosis and initiation of IST as first-line treatment was 23 days (0–180). The analysis of the association of different factors with OS in these patients showed a negative correlation of the time elapsed to initiation of the first course of IST with OS. A delay in treatment initiation of more than 30 days from diagnosis had a significant impact on treatment outcomes (96.3% vs 48.1%; P = .005) (Fig. 1B).
The results of our study show that the OS achieved with first-line treatment can be considered optimal, with poorer outcomes in terms of EFS. Our findings were consistent with those of previous case series.4–6 One third of the patients in our series did not require allogeneic transplantation and could be rescued with IST in the absence of an HLA-identical related donor. We also found that HSCT as second-line treatment was a good salvage therapy option after 1 course of IST, with a survival greater than 60%. We ought to highlight that the outcomes found in different groups and lines of treatment should not be interpreted with the aim of improving treatment selection, as the retrospective design of this case series only allows the description of what happened with each line of treatment as opposed to an applicable comparison (Table 1).
Characteristics of the total cohort of patients and their treatment.
| N | 48 |
| Age at diagnosis (years) | 11 (8−14) |
| Median duration of follow-up (months) | 36 (13−79) |
| Sex | |
| Male (%) | 33 (69%) |
| Female (%) | 15 (31%) |
| Diagnosis | |
| Acquired aplasia (%) | 33 (68.8%) |
| RCC (%) | 15 (31.3%) |
| IST as first-line, total (%) | 37 (77.08%) |
| ATGAM (%) | 21 (43.75%) |
| Thymoglobulin (%) | 16 (33.33%) |
| Severe neutropenia | 21 (56.7%) |
| Transfusion dependence | 24 (68.8%) |
| Spontaneous remission | 1 (2.08%) |
| Progression to PNH | 1 (2.08%) |
| HSCT, total (%) | 20 (41.6%) |
| HSCT as first-line (HLA-identical related donor) (%) | 9 (18.75%) |
| HSCT > first line (%) | 11 (22.91%) |
| Conditioning | |
| Myeloablative | 5 (25%) |
| Reduced toxicity | 15 (75%) |
| Outcome of patients that underwent HSCT | |
| No GvHD | 14 (70%) |
| Acute GvHD | 5 (25%) |
| Chronic GvHD | 1 (5%) |
| In complete remission (%) | 16 (80%) |
| Deceased (%) | 4 (20%) |
| Outcomes of patients treated with IST | |
| In complete remission (%) | 17 (35.4%) |
| In treatment with CsA (%) | 7 (18.9%) |
| Posterior HSCT (%) | 11 (29.7%) |
| Death during IST (%) | 1 (2.7%) |
| Lost to follow-up (%) | 1 (2.7%) |
Ranges given as Q1-Q3.
ATGAM, equine anti-thymocyte immune globulin; CMV, human cytomegalovirus; CsA, cyclosporine A; GvHD, graft versus host disease; HLA, human leukocyte antigen; HSCT, haematopoietic stem cell transplantation; IST, immunosuppressive therapy; PNH, paroxysmal nocturnal haemoglobinuria; RCC, refractory cytopenia in childhood.
At present, the appropriateness of using HSCT for first-line treatment of these patients independently of the type of donor available is being investigated due to the excellent outcomes observed in patients managed with this approach, as was the case in our series with treatment with HLA-matched related donor HSCT. However, we think it is important to take into account that as many as a third of the patients may achieve a cure with IST alone. Although it may be even more important to underscore that, if IST is used as first-line treatment, it should be initiated within 30 days of diagnosis to avoid a negative impact on its outcomes.
Previous presentations: This study was presented in the LXIII National Congress of the Sociedad Española de Hematología y Hemoterapia and the XXXVII National Congress of the Sociedad Española de Trombosis y Hemostasia, held in 2021 in Pamplona, Spain.