Eosinophilic oesophagitis (EoE) affects the quality of life (QoL) of the patients and their families. Specific quality of life questionnaires help to evaluate the QoL for this particular disease, as well as to make diagnostic or therapeutic changes in order to improve it.
ObjectiveTo make a transcultural adaptation of the original English version of the Pediatric Eosinophilic Esophagitis Quality of Life Module (PedsQL EoE Module) into the Spanish language.
MethodsThe PedsQL EoE Module questionnaire consists of 3 versions for parents and children from 5–7, 8–12, and 13–18 years, and one for parents of children from 2 to 4 years. It follows the international consensus methodology, consisting of performing independent translations of the original English version to Spanish, a consensus Spanish version, a back-translation into English, preliminary final version, as well as a cognitive interview with 5 parents and children of each age group in order to evaluate the comprehension of the questionnaire, and once the difficulties found are resolved, the final version.
ResultsIn the cognitive interview, 15 children and 20 parents responded to all the questions. The time taken to complete the questionnaire seemed adequate to them, and the comprehension test was easy. The 5–7 years children group took the longest in responding and had more difficulty in understanding it. The overall satisfaction was high, and the questionnaire seemed to be a useful tool for them.
ConclusionsAfter its validation, the transcultural adaptation to Spanish of the PedsQL EoE Module questionnaire can be applied in order to study the QoL of Spanish children with EoE.
La esofagitis eosinofílica (EoE) afecta a la calidad de vida (CVRS) de los pacientes y sus familias. Los cuestionarios de calidad de vida específicos permiten evaluar la CVRS para esa enfermedad concreta y realizar cambios diagnósticos o terapéuticos para mejorarla.
ObjetivoRealizar la adaptación transcultural de la versión inglesa original del cuestionario Pediatric Eosinophilic Esophagitis Quality of Life Module (Peds QL EoE Module) a lengua española.
MétodosEl cuestionario Peds QL EoE Module consta de 3 versiones para padres y niños de 5-7, 8-12 y 13-18 años y una para padres de niños de 2-4 años. Se siguió la metodología internacionalmente consensuada, realizando 2 traducciones independientes de la versión original inglesa al español, versión española de consenso, traducción inversa al inglés, versión final preliminar, entrevista cognitiva a 5 padres y niños de cada grupo de edad para evaluar la comprensión del cuestionario y crear, una vez resueltas las dificultades encontradas, la versión final.
ResultadosEn la entrevista cognitiva respondieron la totalidad de las preguntas 15 niños y 20 padres; el tiempo empleado en contestar el cuestionario les pareció adecuado y el test, de fácil comprensión. El grupo de niños de 5 a 7 años fue el que más tiempo tardó en responder y el que presentó mayor dificultad en su comprensión. La satisfacción global fue alta y la encuesta les pareció una herramienta útil.
ConclusionesLa adaptación transcultural al español del cuestionario Peds QL EoE Module permitirá, tras su validación, su aplicación para el estudio de la CVRS de los niños españoles con EoE.
Eosinophilic oesophagitis (EoE) is a chronic immune disorder limited to the oesophagus and is characterised by symptoms of oesophageal dysfunction accompanied by eosinophilic infiltration with more than 15 eosinophils per high power field in oesophageal biopsy samples.1
In young children, EoE manifests with regurgitation, vomiting, food refusal or faltering growth. In older children and adolescents, the most frequent symptoms are dysphagia, food impaction, retrosternal chest pain, abdominal pain and/or heartburn. Performance of gastroscopy with obtention of biopsy samples is necessary for diagnosis and to evaluate the response to treatment.2
Once the diagnosis is confirmed, there are 3 treatment options: administration of proton pump inhibitors, administration of oral corticosteroids or elimination of suspect foods from the diet. Effective treatment is defined as treatment achieving histological remission and should be maintained indefinitely to control the disease.1,2
The diagnosis of EoE has an impact on the quality of life of affected patients due to its chronic course, the functional impairment and social limitations it causes and the numerous health care visits, multiple tests and permanent pharmacological or dietary treatment that it requires.3,4
The assessment of health-related quality of life (HRQoL) is one of the objectives of health care teams in the comprehensive evaluation of the patient. The first instruments developed to this end were questionnaires for assessment of generic HRQoL. One of the most widely used ones is the Pediatric Quality of Life Inventory (PedsQL),5 which has been used to assess HRQoL in healthy children as well as children with various diseases. However, these questionnaires did not assess specific aspects of each disease that could have an impact on the HRQoL of affected patients, which led to the subsequent development of specific questionnaires for different diseases.
Taft et al.6 were the first to develop a specific questionnaire for adult patients with EoO (the Adult Eosinophilic Oesophagitis Quality of Life [EoO-QoL-A]), a Spanish translation and transcultural adaptation of which was made and validated by Lucendo et al.7
In 2012, Franciosi et al.8–10 developed the specific questionnaire Pediatric Eosinophilic Esophagitis Quality of Life Module (PedsQL EoE Module) for children with EoE and their parents in the United States. This questionnaire meets validity, reproducibility and feasibility criteria and has exhibited a good correlation when compared to the generic PedsQL.5 At present, there is no HRQoL questionnaire in Spanish specific for EoE in the paediatric population. Given the difficulty involved in developing a new questionnaire from scratch, we selected the PedsQL EoE Module because it had been developed with rigorous methods and by a group with ample experience on the subject.
The aim of our study was to produce a translation and transcultural adaptation of the PedsQL EoE Module to Spanish, achieving semantic and content equivalence with the original source instrument in English, to make available a specific instrument to assess HRQoL in children with EoE in Spain.
MethodsWe conducted a qualitative study in which we did a transcultural adaptation of the PedsQL EoE Module. To do so, we needed to gather a group of patients aged 5–18 years and a group of parents of children aged 2–18 years to assess the semantics and syntax of each item and obtain the necessary feedback for developing the final version of the questionnaire. To ensure that the sample was representative, we contacted a group of patients with EoE and their parents through the Asociación Española de Pacientes con Esofagitis Eosinofílica (Spanish Association of Patients with Eosinophilic Oesophagitis, AEDESEO) by electronic mail or telephone. We selected patients that accepted to participate by consecutive sampling until we achieved the required sample size established by the Mapi Research Trust. We only made occasional contact with a few patients of the paediatric gastroenterology clinic. Interviews were made by direct personal contact with the research team.
Description of the Pediatric Quality of Life Eosinophilic Esophagitis ModuleThis questionnaire has 33 items organised in 7 scales, as shown in Table 1.
Description of the scales of the PedsQL EoE Module.
| Scale | Description | Number of items |
|---|---|---|
| Symptoms I | Gastrointestinal symptoms | 6 |
| Symptoms II | Problems swallowing | 4 |
| Treatment | Problems related to treatment | 5 |
| Worry | About the disease, diagnostic tests and treatment | 6 |
| Communication | Trouble talking about the disease | 5 |
| Food and eating | Food allergy and dietary restrictions | 4 |
| Food feelings | Related to dietary restrictions | 3 |
There are 2 versions of the PedsQL EoE Module based on the recall period: a standard version to collect information on the symptoms, treatment, worry, food and eating and feelings in the past month, and an acute version to collect information referring to the past week.
Each questionnaire also has 2 different self-administered versions to be completed independent of each other: the paediatric patient self-report and the parent proxy report. There are also different paediatric questionnaire versions for different age groups: 5–7 years, 8–12 years and 13–18 years. There are also different versions of the parent questionnaire for these age groups, and an additional version for parents of children aged 2–4 years.
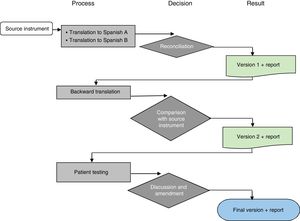
Transcultural adaptationThe transcultural adaptation process adhered to international guidelines and the methodology proposed by the Mapi Research Trust (Fig. 1).11,12
Process of transcultural adaptation of a quality of life questionnaire. Source: Adapted from Mapi Research Trust.12
We contacted the Mapi Research Trust, which currently owns the rights to the PedsQL EoE Module, and obtained their consent to use the questionnaire. The research team committed to adhering to the guidelines for validation proposed by the Mapi Research Trust.
The process involves the following phases:
Forward translationTwo native Spanish translators that were bilingual in English and Spanish made independent translations to Spanish of the original questionnaire in English. Using these 2 versions, the research group developed the initial Spanish version of the questionnaire by consensus. We analysed each item in each scale to assess the semantic and syntactic equivalence with the original.
Backward translationA native English translator bilingual in English and Spanish did the back translation to English of the initial Spanish version of the questionnaire developed by consensus for the purpose of verifying that the contents were faithful to the original.
Once the backward translation was completed, the translator that made it and the research team analysed each of the items to rate the similarity of the Spanish and English versions in terms of semantics and syntax. This process resulted in the preliminary final Spanish version.
Cognitive interviewThe following step was to verify that patients could understand each of the items and correctly interpret their meaning. To do so, we distributed the questionnaires for the applicable age group to 5 parents in each age group (2–4, 5–7, 8–12 and 13–18 years) and 5 patients in each of the 3 higher age groups (after obtaining verbal consent from the parents and consent or assent from the children).
We carried out in-person individual cognitive interviews. We interviewed children and their parents separately to prevent interference with the responses or opinions of the children. We documented comprehension problems as they emerged as well as any adaptations proposed by patients or parents.
Development of the final versionAfter assessing the difficulties and suggestions expressed by children and parents, the research team made the pertinent changes by consensus to develop the final version in Spanish of the PedsQL EoE Module.
Reading testThe final version was read by a parent and a patient that did not raise any objections, so no additional changes were made to it.
We submitted all the accumulated documentation to the Mapi Research Trust to obtain their final approval: the 2 translations to Spanish, the initial consensus-based version, the backward translation to English, the preliminary final version and the definitive final version. We also submitted summaries of the cognitive interviews and the suggestions made by patients and parents, along with the changes made by the research team based on the obtained information, as well as a summary of each of the phases of the process.
Ethical considerationsThe study was approved by the Ethics Committee of the Hospital Universitario de Fuenlabrada.
ResultsQuestionnaire forward and backward translation processIn the consensus process for development of the Spanish version, the main points under discussion were the following:
In children aged 5–7 years, the literal translation of the first answer choice (“not at all”) would be “de ningún modo” or “nada en absoluto”, but we chose to use a simpler term, “nunca” to facilitate comprehension. In the same age group, in the feelings scale, for the question “¿Te preocupa comer algún alimento al que eres alérgico/a o que no deberías comer?” we thought that the expression “te preocupa” may be too abstract and that it would be more understandable for these children if the item read “te da miedo”. In the “how I talk to others” scale, the initial translation was “te cuesta decírselo a…”, but we switched it to “es difícil para ti decírselo a…” to improve comprehension.
Questionnaire comprehensionWe were able to administer the questionnaire to the necessary number of children and parents in each age group. We interviewed a total of 15 patients (13 boys [86.6%] and 2 girls [14.4%]) and 20 parents, of who 14 were mothers. We recruited most parents through the AEDESEO, but we needed to recruit another 2 patients from a primary care caseload and 2 patients from the caseload of the paediatric gastroenterology department of a tertiary care hospital to obtain the necessary number of patients in the younger age group.
The preliminary final version of the questionnaire was completed by every participant during the cognitive interview. All parents and 86% of the children reporting understanding the entire questionnaire well. Forty-six percent of patients and 65% of parents gave suggestions as to the items that needed revising, and 33% of children and 60% of parents suggested specific modifications (Tables 2 and 3).
Questionnaire comprehension and acceptability by age group.
| 13–18 years (n=5) | 8–12 years (n=5) | 5–7 years (n=5) | % global | |
|---|---|---|---|---|
| Easy to understand | 100% | 80% | 60% | 80% |
| Items to change | 20% | 0% | 60% | 26.6% |
| Suggestions for changes | 80% | 0% | 40% | 40% |
| Clear instructions on how to complete | 80% | 80% | 40%60% nk/na | 66.6% (20% nk/na) |
| Adequate time to complete | 100% | 100% | 60% | 86.6% |
| Boring | 0% | 0% | 20% | 6.7% |
| Useful | 100% | 100% | 0%100% nk/na | 66.6%group of 5–7 years: nk/na |
nk/na: not known/no answer.
Questionnaire comprehension and acceptability. Parents.
| 13–18 years | 8–12 years | 5–7 years | 2–4 years | % global | |
|---|---|---|---|---|---|
| Easy to understand | 100% | 100% | 100% | 100% | 100% |
| Items to change | 40% | 60% | 60% | 100% | 65% |
| Suggestions for changes | 60% | 0% | 40% | 100% | 50% |
| Clear instructions on how to complete | 80% | 80% | 60% | 60% | 70% |
| Adequate time to complete | 100% | 100% | 100% | 100% | 100% |
| Boring | 0% | 0% | 0% | 0% | 0% |
| Useful | 100% | 100% | 100% | 100% | 100% |
When it came to the item “Me preocupa ponerme enfermo delante de otras personas” (“I worry about getting sick in front of other people”), older children and parents suggested adding some symptoms related to the disease to improve comprehension, and the final item read “Me preocupa ponerme enfermo/a (vomitar, atascarme) delante de otras personas”. Some children in the 5-to-7 years age group expressed difficulties with negative sentences, which translated literally to Spanish involved the use of double negatives, for things that in Spanish, contrary to English, would more frequently be expressed with a positive sentence. We proposed changing the phrasing of the item to switch it to a positive sentence, but after consulting with the authors of the original questionnaire, and given that this issue did not affect all children, we decided to maintain the construction that was used originally.
Some parents had difficulty identifying the symptoms or feelings of their children, especially those in the youngest age group.
Although the instructions provided before administering the questionnaire specified that the items referred to the symptoms, worries and feelings of the children, some parents wondered whether they had to report their own concerns or those of their children. In addition, while the instructions also specified that the items referred to the past month, both parents and children asked what time in the course of disease the items were referring to.
Questionnaire acceptabilityTo assess the acceptability of the questionnaire, we asked participants whether they found it long, useful or boring. None of the parents found it long, boring or useless. When it came to the children, 13.4% found it long, 6.7% found it boring and 66.6% found it useful; children aged 5–7 years were unable to tell why the questionnaire could be useful (Tables 2 and 3). Children in this age group asked for their parents’ assistance, even to have the items read to them, despite the express request to not have parents interfere with the responses of the patients. This was the group that took longest to complete the questionnaire and that required the greatest number of clarifications.
DiscussionWe carried out the transcultural adaptation to Spanish of the specific PedsQL Eosinophylic Esophagitis Module to allow assessment of the health-related quality of life of Spanish children with EoE. The translation was made following the internationally accepted process of forward and backward translation. After, we assessed the preliminary final version by interviewing patients and their parents with the aim of detecting potential comprehension problems and making the necessary corrections in the definitive version.
The earliest studies that assessed HRQoL in children with EoE13 used the generic PedsQL questionnaire. They found that children with EoE had a poorer quality of life, especially in the social and psychological areas. The interest in improving HRQoL has stimulated the development of specific questionnaires adapted to different chronic diseases that assess particularities of the disease of interest. Franciosi et al. conducted a study to identify the concerns of children with EoE and their parents,9 which was the foundation for the development of a specific EoE questionnaire for the paediatric population.8 There are several versions of this questionnaire for different age groups, which allows adapting the items to the cognitive development of the children. There is also a patient version and a parent proxy version, allowing comparison of the reports of children and the perceptions of the parents, which do not always match.
Most available questionnaires are in English and can only be applied to the population for which they were designed, so their use in a different context requires the translation and transcultural adaptation to the country where it will be applied. Our research group has previous experience in this area, as we performed the transcultural adaptation and validation of a specific questionnaire for assessment of HRQoL in children with coeliac disease developed in the Netherlands (the Coeliac Disease Dutch Questionnaire [CDDUX]).14
The transcultural adaptation process is complex and must rigorously follow a series of strict stages. In our case, the challenges we met during the process involved the need to adapt the terminology of the questionnaire to the cognitive skills of the age group that it was meant for. The group that had the most difficulty completing the questionnaire was the group of patients aged 5–7 years, for whom it was necessary to adapt the terminology, avoiding abstract concepts that were harder to understand.
We succeeded in recruiting the sample size recommended by the Mapi Research Trust, which was particularly challenging when it came to the 2–4 years and 5–7 years age groups, given the lower frequency of disease in younger children, but allowed us to make an adequate analysis of the questionnaire by age group. Older children can understand and express their difficulties more easily than younger children, and therefore it is important to ensure adequate representation of all age groups.
The cognitive interviewing phase was the hardest from a methodological standpoint, but it allowed us to identify difficulties and limitations in the translated version, especially in the group of patients aged 5–7 years.
Some parents of very young children had difficulty answering some of the questions, due to either children not verbalising certain feelings at a young age or to the difficulty of identifying symptoms and moods in this age group.
The difficulties and suggestions for language changes conveyed by older children and parents were particularly useful in improving the comprehension of the questionnaire, especially as pertained the manifestations of disease, and most of this feedback was incorporated in the final version. This achieves comprehension of the different items by the largest possible number of children and parents. We ought to highlight that despite the instructions specifying that the items referred to either the past month or the past week, both children and parents expressed concerns about what point in the course of illness and treatment the items referred to, which may suggest that they have an interest in expressing how they felt when the disease was at its worst.
The problems we encountered in the transcultural adaptation process were similar to those described by Santos et al., who recently published the adaptation to Portuguese of the Pediatric Eosinophilic Esophagitis Symptom Score (PEES).15 As occurred in our study, they mainly found difficulties in comprehension in the youngest children. The syntactic and semantic differences were similar in both studies.
We sent the final version to the company that owns the rights to the questionnaire, which, after reviewing the submitted documentation, proceeded to approve it.
One of the main limitations of the study was that many of the patients that participated in the cognitive interview resided in the Madrid metropolitan area, with a predominance of households of middle-to-high socioeconomic status. Also, while we asked parents of the youngest patients not to interfere with their children's responses, they may have somewhat influenced their answers in their attempt to help them understand the most challenging questions, but it is rare for patients aged 5–7 years not to need any form of assistance. In fact, most paediatric HRQoL questionnaires have been developed for children aged 8–18 years.5,16,17
The next phase in our project is to validate the Spanish version of this questionnaire, assessing its validity and reliability, to allow its use in Spanish children.
In conclusion, we performed the transcultural adaptation to Spanish of the PedsQL EoE Module, which parents and patients considered a useful instrument in relation to this disease. This questionnaire will be useful for assessing the quality of life if paediatric patients with EoO in the different stages of disease and based on the treatment received, and also for making comparisons with children in other countries where the same questionnaire is used.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García-Martínez de Bartolomé R, Barrio-Torres J, Cilleruelo-Pascual ML. Adaptación transcultural del cuestionario Pediatric Eosinophilic Esophagitis Quality of Life Module. An Pediatr (Barc). 2020;92:332–338.