Thyroid hormone resistance (THR) syndrome is a rare inherited disorder caused by changes in the thyroid hormone receptor beta gene (THRB).1 This genetic change results in an abnormal tissue response to thyroid hormones and typically manifests with elevated levels of thyroid hormones (triiodothyronine [T3] and thyroxine [T4]) and inappropriately normal or elevated thyroid-stimulating hormone (TSH) levels.2 The pattern of inheritance can be autosomal dominant or recessive, but is often dominant,3 and the incidence is of 1 in 40 000 live births.1 The prevalence of THR associated with changes in the beta receptor is similar across sexes but varies among ethnic groups.3,4 The clinical presentation varies widely, from asymptomatic to symptoms of hypothyroidism or hyperthyroidism. In cases with a known family history, prenatal diagnosis is possible, facilitating early postnatal monitoring and management.
We present the case of a male neonate diagnosed with THR syndrome who was born at 40 weeks of gestation in an uncomplicated vaginal delivery to a mother aged 36 years.
The mother had a relevant personal and family history of THR (diagnosed in her mother and sister). She was asymptomatic and not managed with medication. Prenatal diagnosis was conducted via chorionic villous biopsy during the first ultrasound at 12 weeks and 3 days of gestation, with no documented complications. The results of genetic testing, obtained at 22 weeks of gestation, confirmed that the fetus carried the familial mutation c.1312C>T (p.Arg438Cys) in exon 10 of the THRB gene. The pregnancy proceeded without complications.
After birth, the 1- and 5-minute Apgar scores were 9 and 10, respectively, and there were no immediate signs of respiratory or cardiovascular distress. The physical examination was unremarkable, with no dysmorphic features or goiter and a birth weight of 3175g (z score, −0.36).
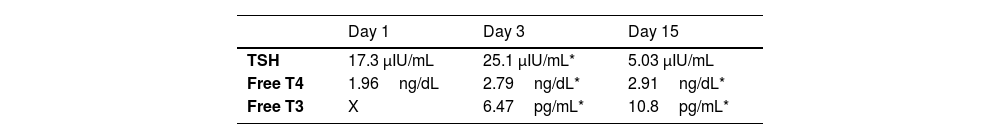
Due to the known mutation, a cord blood specimen was sent for thyroid function testing. The laboratory results showed a free T4 level of 1.96ng/dL (reference range, 0.8–2.2)5 with a TSH level of 17.3mIU/L (reference range, 0.5–10.0mIU/L).5Table 1 presents data on the thyroid function profile during the first month of life.
The neonate was discharged on day 2 post birth under follow-up by the pediatric endocrinology team, with close monitoring of clinical symptoms, growth and thyroid function test results. He remained asymptomatic, feeding well, with good linear growth and weight gain. The findings of the echocardiogram and electrocardiogram on day 27 post birth were normal. Given his stable clinical condition and the absence of symptoms, treatment was not initiated.
Thyroid hormone resistance syndrome poses unique challenges in management, especially in neonates and infants. While most individuals with THR remain asymptomatic, the clinical manifestations can vary widely, which requires individualized monitoring and treatment. In this case, the rare in-utero diagnosis allowed for immediate postnatal monitoring, avoiding unnecessary delays in the identification of potential thyroid dysfunction as well as unnecessary medication.
The primary consideration in managing neonates with THR is distinguishing between true thyroid dysfunction and the physiological state of THR, in which there is elevation of thyroid hormone levels but tissue sensitivity is abnormal. Most asymptomatic neonates, as was the case presented here, do not require immediate treatment. However, ongoing monitoring of thyroid function, growth and neurodevelopment is essential to ensure optimal long-term outcomes. Any signs of delayed growth or developmental concerns should prompt a reassessment of thyroid status and consideration of treatment.
The findings in this case were consistent with typical THR laboratory profiles, in which elevation of free T4 and free T3 levels coexist with nonsuppressed TSH. Although genetic testing confirmed the diagnosis prenatally, the clinical course remained stable. While the penetrance of THR syndrome is high, the phenotypic expression is variable, even for the same THRB variant.4
The absence of clinical symptoms in this case supports the current conservative management approach, with close monitoring as the primary strategy during infancy.
Long-term management of THR requires maintenance of euthyroidism in tissues where resistance to thyroid hormones may be partial. In some cases, supraphysiological doses of thyroid hormone may be needed to suppress TSH and ensure adequate thyroid function in tissues with impaired sensitivity.
In conclusion, this case highlights the importance of the family history in the early diagnosis of genetic conditions such as THR syndrome. This case is unusual, given its in-utero diagnosis, and is one of the few instances in which this disease has been managed in the early months of life. Managing patients with THR, particularly those diagnosed at such early stage, poses significant challenges. Distinguishing between true thyroid dysfunction and the physiological state of resistance is crucial and requires continuous and individualized monitoring. While many asymptomatic neonates, like our patient, do not require immediate treatment, rigorous follow-up of growth and thyroid function is essential to ensure favorable long-term outcomes. Thus, the approach to the care of these patients should not be underestimated, as the complexity and variability of the syndrome demand careful management by a pediatric endocrinology team and lifelong follow-up.