Aplasia cutis congenita (ACC) is a rare congenital malformation that commonly involves the scalp, but can affect pericranium, bone and dura mater. Complications are rare, but can be fatal, so early treatment must be achieved. The treatment remains controversial with no consensus between the conservative and surgical approach. The aim of this study is to describe our experience in the management of ACC.
Materials and methodsRetrospective review of the medical records of all children up to 14 years diagnosed with ACC and treated between 2000 and 2013.
ResultsThere were a total of 22 cases of ACC with lesions ranging from 1cm (0.79cm2) to 14cm (153.94cm2). ACC of the scalp was found in 18 cases, with 3 in extremities and 1 in trunk. Conservative treatment was performed on 9 patients and 13 underwent surgical treatment (8 primary closures, 2 plasties, 2 skin grafts, and 1 skin flap). Two patients died due to complications of other diseases not related with the ACC.
ConclusionsACC is a rare disease that can be fatal. A complete initial assessment to establish early treatment is necessary to prevent this. Surgery should be considered as an initial therapeutic option in defects >4cm (>12.6cm2) as it prevents the risk of fatal complications.
La aplasia cutis congénita (ACC) es una malformación congénita rara que afecta sobre todo al cuero cabelludo, aunque puede afectar al pericráneo, el cráneo y la meninges. Las complicaciones pueden llegar a ser fatales, por lo que es necesario un tratamiento oportuno. El tratamiento sigue siendo controvertido, sin encontrar un consenso entre el abordaje conservador y el quirúrgico. El objetivo de este estudio es describir nuestra experiencia en el manejo de la ACC.
Material y métodosEstudio descriptivo retrospectivo de las historias clínicas de los pacientes menores de 14 años con diagnóstico de ACC, atendidos entre el año 2000 y el 2013.
ResultadosVeintidós casos de ACC con lesiones que variaban de 1cm (0,79cm2) a 14cm (153,94cm2). Dieciocho casos presentaron lesiones en el cuero cabelludo, 3 en extremidades y uno en tronco. Se realizó tratamiento conservador en 9 y quirúrgico en 13 (8 cierres primarios, 2 plastias, 2 injertos cutáneos y un colgajo). Dos pacientes fallecieron por complicaciones de otras patologías no asociadas a la ACC.
ConclusionesLa ACC es infrecuente y puede tener un desenlace fatal. Para prevenirla es necesaria una evaluación inicial completa para establecer un tratamiento oportuno. La cirugía es una buena opción terapéutica, sobre todo en defectos con diámetro>4cm (12,6cm2), ya que disminuye el riesgo de complicaciones mortales.
Aplasia cutis congenita (ACC) is a malformation consisting of the congenital absence of skin in certain areas. It most commonly involves the scalp, with a depth confined to the epidermis or dermis, and the appearance of a scar or ulcer. Its aetiology is unknown, although there are multiple biological, pharmacological, mechanical and genetic factors that could contribute to its aetiopathogenesis. A pattern of genetic inheritance has been described in some cases, in which ACC was associated to other congenital defects.1
The diagnosis is usually made when the newborn is first examined. Its treatment varies according to the size and localisation of the defect and the degree of involvement of adjacent structures. Early treatment is recommended to prevent complications such as sagittal sinus haemorrhage, infection, fluid and electrolyte imbalances, or thermal imbalances.2
The aim of this study was to describe the clinical and epidemiological characteristics of ACC, as well as our experience in its management.
Materials and methodsWe conducted a descriptive and retrospective study by reviewing the medical records of patients with ACC aged less than 14 years that were treated in our department between January 2000 and December 2013. We included every patient with a presurgical diagnosis compatible with ACC. We excluded patients with lesions in which the microscopic findings of the histological examination were incompatible with ACC. We performed a descriptive analysis of the variables based on their distribution and using measures of central tendency, assessing the associations between the treatment used, the size of the lesion, the development of postoperative complications, the length of stay and the need for reoperation.
Conservative management of patients included daily local therapy with topical silver sulfadiazine 1% or nitrofurazone 0.2% and application of an occlusive dressing until secondary-intention healing was complete. Several approaches were used for surgical management, including excision and primary closure of the defect, full-thickness skin grafting, split-thickness grafting following the previous implantation of biosynthetic primate acellular matrix or bilayer matrix products, and rotation or advancement flaps, with or without the use of tissue expanders. The surgeon in charge determined whether a conservative or surgical approach was indicated. This decision was made based on lesion size, bone involvement and the medical condition of the patient.
Whenever biological samples were obtained, they were sent for histopathological examination, and whenever a patient died, an autopsy was performed to determine the cause. In cases in which biological samples were not obtained, the diagnosis was made based on the clinical manifestations. Patients were followed up in the outpatient clinics to monitor the surgical wound or skin lesion and assess for comorbidities, postoperatory complications and cosmetic outcomes.
ResultsWe reviewed the records of 23 patients that received an initial diagnosis of ACC, and excluded one case in which the histological examination of the surgical specimen was not compatible with ACC (nevus sebaceous of Jadassohn).
Twenty-two patients with ACC received treatment (Table 1). In 54.5% (12/22) of the patients, diagnosis was made at birth with immediate initiation of treatment. The rest of the patients (10/22) received the diagnosis at birth, but were referred for treatment at ages ranging from 4 months to 13 years, with a median age of 32 months (IQR, 12–111 months).
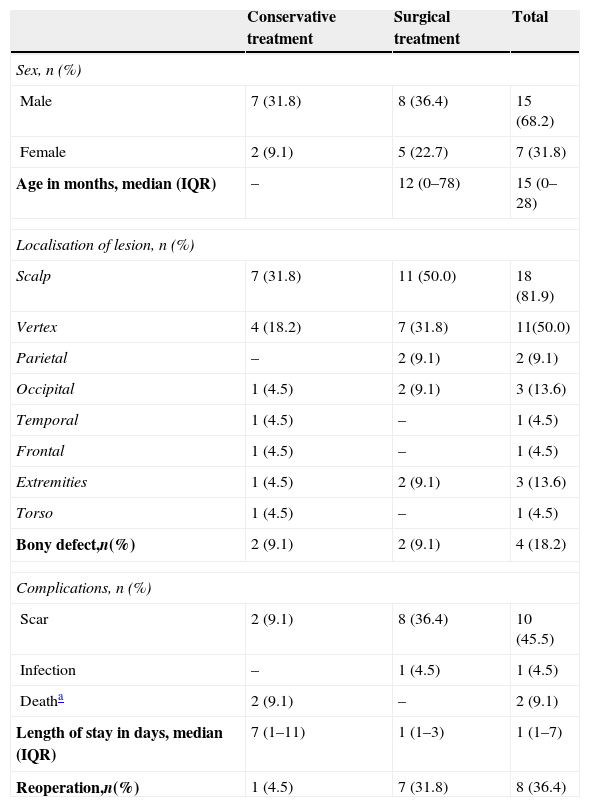
Comparison of surgical and conservative treatment of aplasia cutis congenita, (n=22).
| Conservative treatment | Surgical treatment | Total | |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 7 (31.8) | 8 (36.4) | 15 (68.2) |
| Female | 2 (9.1) | 5 (22.7) | 7 (31.8) |
| Age in months, median (IQR) | – | 12 (0–78) | 15 (0–28) |
| Localisation of lesion, n (%) | |||
| Scalp | 7 (31.8) | 11 (50.0) | 18 (81.9) |
| Vertex | 4 (18.2) | 7 (31.8) | 11(50.0) |
| Parietal | – | 2 (9.1) | 2 (9.1) |
| Occipital | 1 (4.5) | 2 (9.1) | 3 (13.6) |
| Temporal | 1 (4.5) | – | 1 (4.5) |
| Frontal | 1 (4.5) | – | 1 (4.5) |
| Extremities | 1 (4.5) | 2 (9.1) | 3 (13.6) |
| Torso | 1 (4.5) | – | 1 (4.5) |
| Bony defect,n(%) | 2 (9.1) | 2 (9.1) | 4 (18.2) |
| Complications, n (%) | |||
| Scar | 2 (9.1) | 8 (36.4) | 10 (45.5) |
| Infection | – | 1 (4.5) | 1 (4.5) |
| Deatha | 2 (9.1) | – | 2 (9.1) |
| Length of stay in days, median (IQR) | 7 (1–11) | 1 (1–3) | 1 (1–7) |
| Reoperation,n(%) | 1 (4.5) | 7 (31.8) | 8 (36.4) |
IQR, interquartile range.
All scalp lesions were solitary, except in one patient that had two adjacent lesions at the vertex level. The diameter of the lesions ranged from 1cm (area, 0.79cm2) to 14cm (area, 153.94cm2), with a median diameter of 3.5cm (IQR, 2–7cm) to an area of 9.81cm2 (IQR, 3.14–38.48cm2). The lesions in the extremities consisted of lesions 2cm in diameter (area, 3.14cm2) in the third finger of the right hand, 3cm in diameter (area, 7.07cm2) in both hands, and 2cm in diameter on the back of both hands and both legs. The lesions in the patient with systemic involvement were large, and affected approximately 20% of the total body surface area, especially the torso and the upper limbs.
Five patients with ACC in the scalp had associated diseases that included: heart disease with ventricular septal defect, duplex collecting system, complete cleft palate with Pierre-Robin sequence, Adams-Oliver syndrome, and one girl with multiple malformations. The other patients had dystrophic epidermolysis bullosa, Streeter dysplasia and Bart syndrome. Genetic testing was performed in only two patients, and did not detect chromosomal abnormalities in either.
Scalp lesions were managed with conservative treatment in seven patients (36.3%), all of whom had lesions smaller than 4cm (12.57cm2), except for one lesion with a 9cm diameter (area, 63.62cm2) that was managed conservatively due to high anaesthetic and surgical risk. The rest of the patients were treated with surgery (Table 2). The patient with a lesion of 14cm (154cm2), with an associated bony defect of similar size underwent implantation of a biosynthetic matrix (INTEGRA® Matrix Wound Dressing, Integra LifeSciences Corporation, Plainsboro, New Jersey, USA) followed by a split-thickness graft to cover the defect. The second patient with a large bony defect underwent primary closure of the scalp with no additional treatment on the bone, and responded favourably. The other two patients that had bony defects were managed conservatively, and showed good bone development and closure of the defect.
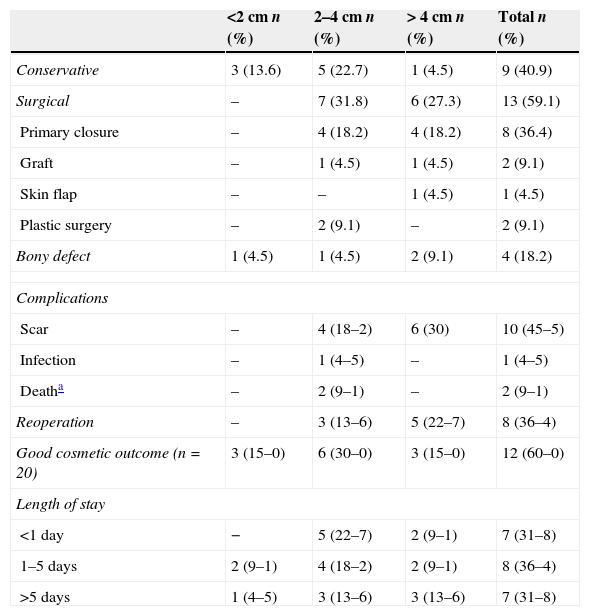
Comparison by aplasia cutis congenita lesion size (n=22).
| <2cm n (%) | 2–4cm n (%) | > 4cm n (%) | Total n (%) | |
|---|---|---|---|---|
| Conservative | 3 (13.6) | 5 (22.7) | 1 (4.5) | 9 (40.9) |
| Surgical | – | 7 (31.8) | 6 (27.3) | 13 (59.1) |
| Primary closure | – | 4 (18.2) | 4 (18.2) | 8 (36.4) |
| Graft | – | 1 (4.5) | 1 (4.5) | 2 (9.1) |
| Skin flap | – | – | 1 (4.5) | 1 (4.5) |
| Plastic surgery | – | 2 (9.1) | – | 2 (9.1) |
| Bony defect | 1 (4.5) | 1 (4.5) | 2 (9.1) | 4 (18.2) |
| Complications | ||||
| Scar | – | 4 (18–2) | 6 (30) | 10 (45–5) |
| Infection | – | 1 (4–5) | – | 1 (4–5) |
| Deatha | – | 2 (9–1) | – | 2 (9–1) |
| Reoperation | – | 3 (13–6) | 5 (22–7) | 8 (36–4) |
| Good cosmetic outcome (n=20) | 3 (15–0) | 6 (30–0) | 3 (15–0) | 12 (60–0) |
| Length of stay | ||||
| <1 day | − | 5 (22–7) | 2 (9–1) | 7 (31–8) |
| 1–5 days | 2 (9–1) | 4 (18–2) | 2 (9–1) | 8 (36–4) |
| >5 days | 1 (4–5) | 3 (13–6) | 3 (13–6) | 7 (31–8) |
Of the four patients that had lesions in the extremities, two underwent surgery. In one, several full-thickness grafts were inserted in the lesions of the hands, and a series of plastic surgeries were performed on the patient that had the lesion in the finger. A biopsy was performed on the patient that had lesions on the hands and feet, confirming the ACC diagnosis; conservative management was chosen for this patient. The patient with torso involvement received conservative treatment.
The median length of stay was less than 1 day (IQR, 0.5–7), with a maximum of 25 days; patients were followed up for a median of 2 years and 7 months. Two patients died in our study (9.52%); the first was a newborn girl aged 11 days with a polymalformative syndrome with complex heart disease that developed heart failure and a low-output intestinal fistula; the second was a patient with Bart syndrome that died from haemodynamic decompensation resulting from the large size of the epidermolysis bullosa lesions.
DiscussionAplasia cutis congenita is a rare disease characterised by the localised absence of skin, and its incidence rate is of 1–3 per 10000 live births per year.3,4 Its specific causes are not known, although it is assumed that the lesion develops in utero due to abnormal ectodermal development in the embryonic period secondary to a mechanical, vascular, teratogenic or hereditary disturbance, unrelated to the delivery or first hours post birth.5 It has been described in association with isolated malformations such as patent ductus arteriosus, tracheoesophageal fistula, epidermolysis bullosa, limb malformations, cleft lip and palate, and kidney malformations, among others.6,7 In our series, 22% of the patients had associated congenital malformations, including one case of Adams-Oliver, one of Streeter dysplasia and one of Bart syndrome.
Ultrasound examination in the last weeks of gestation may lead to prenatal suspicion of ACC,8 although the condition is diagnosed by examination of the newborn.9 In our series, 47.5% of the patients were referred to our clinic more than four months post birth, even though the initial diagnosis had been made in the neonatal period. We believe that an evaluation should be performed and an individualised treatment plan developed immediately after diagnosis. If the diagnosis is uncertain, a biopsy of the lesion could be performed to rule out other dermatoses.10 Additional testing is recommended to rule out associated anomalies.6
Mortality due to haemorrhage, sagittal sinus thrombosis or central nervous system infection can reach up to 50%.2,10,11 Mortality is associated with larger lesion sizes and underlying bony defects. Therefore, treatment must be geared towards the prevention of these complications. In our experience, mortality was not associated with ACC complications, but to complications of associated pathologies.
The typical presentation consists of a solitary lesion localised to the scalp vertex, oval in shape, well demarcated, hairless, with the longest diameter ranging between 1 and 3cm (area, 0.79–7.07cm2), and the appearance of scar or granulation tissue.10,12 Seventy to ninety percent of cases involve the scalp, and up to thirty percent are associated with bony defects.13
There is great controversy surrounding the treatment of ACC, and there is no consensus as to the treatment approach or type of intervention that should be implemented.2 In our experience, there are limited possibilities for adequate closure of the scalp due to its low elasticity, restricted mobility, spherical shape requiring longer skin flaps and limited tissue availability due to the need for preserving axial blood flow. Therefore, the therapeutic approach must be based on the size of the lesion and the presence or absence of underlying cranial defects.9,14 Tissue specimens obtained during surgery should be submitted for histopathological examination to rule out other dermatoses, including nevus sebaceous, traumatic scarring, scarring alopecia, epidermolysis bullosa, localised infection, congenital dermoid cysts, cutaneous meningioma and heterotopic brain tissue, among others.10
Lesions with a diameter of less than 2cm (area, 3.14cm2) and no osseous involvement can be treated conservatively.2,15 The cosmetic outcomes are good, the risk of complications is low, and anaesthesia is not required.16 Another possibility is to perform a radical excision of the lesion under local anaesthesia and sedation, followed by primary closure.
Defects between 2 and 4cm in diameter (area, 3.14–12.57cm2) can be managed conservatively,17 although primary closure of the defect is preferred if the defect is larger than 2cm and with exposed bone.
In cases involving lesions larger than 4cm or bony defects, the conservative approach is not recommended due to the risk of complications, poor cosmetic outcomes and the need for longer hospitalisations. Among the surgical options that have been described in the literature,11 the one we performed most often was primary closure in a single or a series of surgeries. Treatment with skin grafts, dermal substitutes and skin flaps yielded good results and has been described extensively in other published case series.2,6,14
Surgical treatment is preferred in cases associated with bony defects.11 The use of skin flaps has been described in the literature, and this technique has shown very good outcomes and an associated decrease in the risk of complications.5,6,14 Other options include full-thickness grafts, split-thickness grafts and tissue expansion.18 There have also been reports of the use, with favourable outcomes, of allografts,19 acellular dermal grafts and cultured keratinocyte grafts.20
Lesions in the torso or extremities may be managed conservatively.21 If the lesion is very large or it involves a joint, surgical treatment with skin grafts or plastic surgery is recommended to avoid functional limitations or very large scars.
Complications following conservative or surgical treatment may include hypertrophic scarring and keloids with or without cicatricial alopecia and secondary infection of the wound.2,10 In our case series, the most frequent complication was scarring, and it usually did not require additional surgeries.
A broad range of therapeutic approaches are still being used for ACC. Due to the characteristics of this study, we were unable to determine which treatment is best for ACC, but surgical management is usually preferred, especially for larger lesions, as it can prevent fatal complications and the cosmetic results are good.
ConclusionsWe believe that ACC is a disease that carries the risk of potentially fatal complications, but that these can be prevented with appropriate treatment. While conservative management is an option, we favour surgery as long as it can be performed on the patient. Surgery, regardless of which technique is used, must be performed with the goal of closing the skin defect, and should be considered especially for lesions more than 4cm in diameter or with associated bony defects.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Betancourth-Alvarenga JE, Vázquez-Rueda F, Vargas-Cruz V, Paredes-Esteban RM, Ayala-Montoro J. Manejo quirúrgico de la aplasia cutis congénita. An Pediatr (Barc). 2015;83:341–345.