Advances in molecular diagnosis have made it possible to detect previously unknown viral agents as causative agents of lower respiratory tract infections (LRTI). The frequency and relevance of viral coinfections is still debatable.
ObjectiveCompare clinical presentation and severity between single virus infection and viral coinfection in children admitted for LRTI.
MethodsA 3-year period observational study (2012–2015) included children younger than two years admitted for LRTI. Viral identification was performed using PCR technique for 16 viruses. Clinical data and use of health resources was gathered during hospital stay using a standard collection form and we compared single virus infection and viral coinfections.
ResultsThe study included 524 samples (451 patients); 448 (85.5%) had at least one virus identified. Viral coinfections were found in 159 (35.5%). RSV and HRV were the most commonly identified virus; bronchiolitis and pneumonia the most frequent diagnosis. Patients with viral coinfections were older, attended day-care centers, had previous recurrent wheezing more frequently and were more symptomatic at admission. These patients did not have more complementary exams performed but were prescribed medications more often. Viral coinfection group did not show longer length of hospital stay and oxygen need, more need for ICU nor ventilatory support.
DiscussionOur study showed a significant proportion of viral coinfections in young infants admitted with LRTI and confirmed previous data showing that prescription was more frequent in inpatients with viral coinfections, without an association with worst clinical outcome.
Avances en el diagnóstico molecular han hecho posible la detección de agentes virales desconocidos en infecciones de las vías respiratorias inferiores (IVRI). Sin embargo, sigue habiendo dudas relativamente a su frecuencia y relevancia.
ObjetivoComparar la clínica y la gravedad entre la infección por virus único y la coinfección en niños admitidos por IVRI.
MétodosSe realizó un estudio durante 3 años consecutivos (2012-2015) que incluyó a niños menores de 2 años ingresados por IVRI. La identificación viral se realizó mediante la técnica de PCR para 16 virus. Los datos clínicos y el uso de los recursos hospitalarios se recogieron de forma estándar durante la estancia hospitalaria y se compararon la infección única con coinfecciones virales.
ResultadosFueron analizadas 524 muestras (451 pacientes); 448 (85,5%) tuvieron al menos un virus identificado. Coinfecciones virales se encontraron en 159 (35,5%). RSV y HRV fueron los virus más frecuentes; bronquiolitis y neumonía, los diagnósticos principales. Los pacientes con coinfecciones virales eran mayores, iban a la guardería, tenían sibilancias recurrentes con más frecuencia y eran más sintomáticos al ingreso. No fueron sometidos a más exámenes, pero les fueron prescritos medicamentos con más frecuencia. El grupo de la coinfección viral no mostró una mayor duración de la estancia hospitalaria, de la necesidad de oxígeno, de UCI o soporte ventilatorio.
DiscusiónNuestro estudio mostró una proporción significativa de coinfecciones virales en los niños pequeños ingresados con IVRI y confirma dados previos que muestran que la prescripción es más frecuente en las coinfecciones virales, sin asociación con peor resultado clínico.
Lower respiratory tract infections (LRTI) are a common cause of hospital admission in pediatric patients,1–3 especially under two years of age.4–6 Different viruses have been identified as etiologic agents of LRTI [respiratory syncytial virus (RSV), adenovirus (ADV), influenza virus A (FLUA) and B (FLUB), human rhinovirus (HRV), parainfluenza1 (PIV1), 2 (PIV2), 3 (PIV3)].7–10 RSV remains the most important viral pathogen in infancy both as a single agent and in coinfection.4,6
In the last two decades, the emergence of polymerase chain reaction (PCR) based technology has substantially increased the sensitivity for viral diagnosis, detecting a larger number of viruses, including more than one virus in the same respiratory secretion sample.2,11,12 Such assays identify virus not growing in standard tissue culture,2 and have identified at least four new viruses associated with LRTI: metapneumovirus (MPV),13 coronavirus (COV),14–16 bocavirus (HBOV)17 and parainfluenza 4 (PIV4).18,19
In children hospitalized with severe LRTI, viral coinfection detected by PCR shows a prevalence ranging between 14% and 44%.20 Whether viral coinfection has a significant clinical impact is yet to be defined.6,11,21–24 A recent meta-analysis found divergent outcomes dependent mainly on age (adult patients included), comorbidities, seasonality, geographical region and methods of detection employed.25
The primary objective of this study was to compare severity of presentation and short-term clinical evolution between single virus infection and viral coinfection in children admitted for LRTI. We additionally intended to compare the amount of health resources used between groups.
We hypothesized that patients with viral coinfection would have a more severe disease and a higher need for diagnostic and therapeutic health care resources.
MethodsThis was a single-center prospective observational study enrolling sequential patients admitted for LRTI, from October 2012 to September 2015, at the Department of Pediatrics of a tertiary care, University affiliated Hospital. This Department receives previously healthy children attending the emergency department (ED) from the geographic area of the hospital and from other local hospitals for chronic and/or severe disease. During the study period, a total of 119,682 children were seen at the ED, from which 3.3% were admitted as inpatients.
Children aged less than two years old with the diagnosis of LRTI were enrolled. The discharge diagnosis was made by the attending physician, who also collected the clinical data by fulfilling a standardized written form, created specifically for the study, at patient's admission and discharge. We analyzed patient's demography (gender, age), comorbidities or associated chronic conditions (prematurity, very low birth weight, respiratory, cardiac, neurologic or genetic), clinical and environmental data (breast-feeding, passive smoke exposure, day care attendance), duration of symptoms and signs related to the acute respiratory infection, and physical examination on admission. Furthermore, we compared the amount of health resources used, namely diagnostic tests performed [including blood analysis, bacteriological cultures of respiratory specimens, chest X-ray (CXR)], treatments prescribed (bronchodilators, antibiotics, oxygen delivery and ventilatory support) and hospital length of stay (LOS) and intensive care unit (ICU) admission.
Respiratory secretions, nasopharyngeal aspirate (NPA) or a nasopharyngeal swab, were obtained within 72h of the child's admission. The strains were sent to the Microbiology Laboratory where the viral and bacteriological studies were carried out. Viral identification was performed through qualitative reading of a real-time PCR technique (Anyplex™ IIRV16 Seegene Inc, till early December 2015 and Allplex Respiratory Pannel I, II e III Seegene Inc, afterwards), both allowing for the simultaneous identification of 16 viruses: ADV, FLUA, FLUB, PIV1, 2, 3 and 4, HRV, RSVA and RSVB, HBOV, COV 229E, NL63 and OC43, MPV and enterovirus (HEV), the second kit with further differentiation of influenza A(H1N1)pdm09 and A(H3).
Viral coinfection was defined when multiple simultaneous viral pathogens were detected from the same respiratory sample and bacterial coinfection or secondary bacterial pulmonary infection based on positive bacteria cultures. Bacterial diagnostic tests were performed only if requested by attending physicians. Strains isolated from products of the respiratory system (expectoration, bronchial secretions) came as samples from a swab, based on the presence of epithelial cells, neutrophils and the morphology of the predominant bacteria. For all types of samples, macroscopically distinct colonies were isolated in pure culture.26 Coinfection was admitted based on microbiological definitions because of the overlap between symptoms and clinical diagnosis, mainly of bronchiolitis and pneumonia.27
Disease severity was analyzed through hospital LOS in days, ICU admission and need for ventilatory support, both invasive and non-invasive.
Statistical analysisFor the purpose of the study all data is referred to as cases or samples. Data was codified before statistical analysis, which was performed using Statistical Package for the Social Sciences® version 20.0. Descriptive statistics were generated: values are expressed as absolute numbers and percentages for discrete variables and as median and interquartile range (IQR) or as mean and standard deviation (SD) for continuous variables, where appropriate. Continuous variables were compared using a two-sided, non-parametric Wilcoxon rank sum test or, when the data were normally distributed (on the basis of the Shapiro–Wilk test), a two-sided Student's t-test. For discrete variables, comparison between groups was performed using contingency table analysis and the Chi-square test or Fischer's exact test as appropriate. All p values were 2-tailed, p<0.05 was considered statistically significant.
A multiple regression model was done to identify clinical and virologic variables associated with hospital LOS. Univariate and multivariate logistic regression models were used to identify variables associated with ICU admission. Factors were selected for inclusion in the model if they were found to be associated with the outcome in unadjusted analyses (p<0.05) or were potentially clinically significant. Results from logistic models were described by odds ratios (OR) and 95% confidence intervals (CI).
Ethics statementThis study was conducted according to the principles expressed in the Declaration of Helsinki, and approved by the Hospital's Ethics Committee. Informed oral consent was obtained from all the parents or the legal responsible.
ResultsGeneral demography and clinical characterizationA total of 524 samples from children aged less than 24 months admitted for LRTI were collected during the study period.
These samples were collected from a total of 451 children, mainly male (n=253; 56.1%), whom 105 (23.3%) were preterm, 112 (24.8%) weighted less than 2500g at birth and 83 (18.4%) had an underlying chronic condition. Fifty-one patients were admitted more than once for an episode of LRTI during the three-year period, and, of these, 19 (37%) had chronic disease.
Overall median (IQR) age of children at episodes was 3.9 (1.2–10.0) months, about half (50.6%) were less than 4 months of age. Day care attendance was reported in 112/442 (25.3%) cases and passive smoke exposure in 214/487 (43.9%).
For the 524 cases, mean (SD) duration of symptoms previous to admission was 5.4 (5.7) days. On admission, cough (466; 88.9%) and rhinorrhea (458; 87.4%) were the main symptoms and respiratory distress (458; 87.4%) the most frequent sign, with hypoxemia found in 171 (32.6%) episodes.
Blood samples were collected and analyzed for complete blood count and C-reactive protein (CRP) in 364 (69.5%) cases, bacteriologic cultures of respiratory secretions in 260 (49.6%) and CXR performed in 343 (65.5%).
During the admission period, supplemental oxygen was prescribed as needed in 472 (90.1%) cases, with a median (IQR) need of 96 (48–144) hours. Nasogastric tube feeding was used in 332 (63.4%) cases. In 320 cases (61.1%) an intravenous antibiotic was prescribed, in the majority (300; 93.8%) due to suspicious or confirmed bacterial infection, including acute otitis media and/or bronchitis or pneumonia. Median (IQR) hospital LOS was 6 (4–9) days.
ICU admission occurred in 116 (22.1%) cases, with a median (IQR) duration of admission in ICU of 4 (3–7) days. In 98 (84.5%) cases ventilatory support was needed: in 34 invasive ventilation, in 49 non-invasive and in 15 cases both. Median (IQR) duration of ventilation was 4 (2–6) days.
Acute bronchiolitis with or without bacterial superinfection was the main discharge diagnosis (328; 62.6%), followed by community-acquired pneumonia (109; 20.8%).
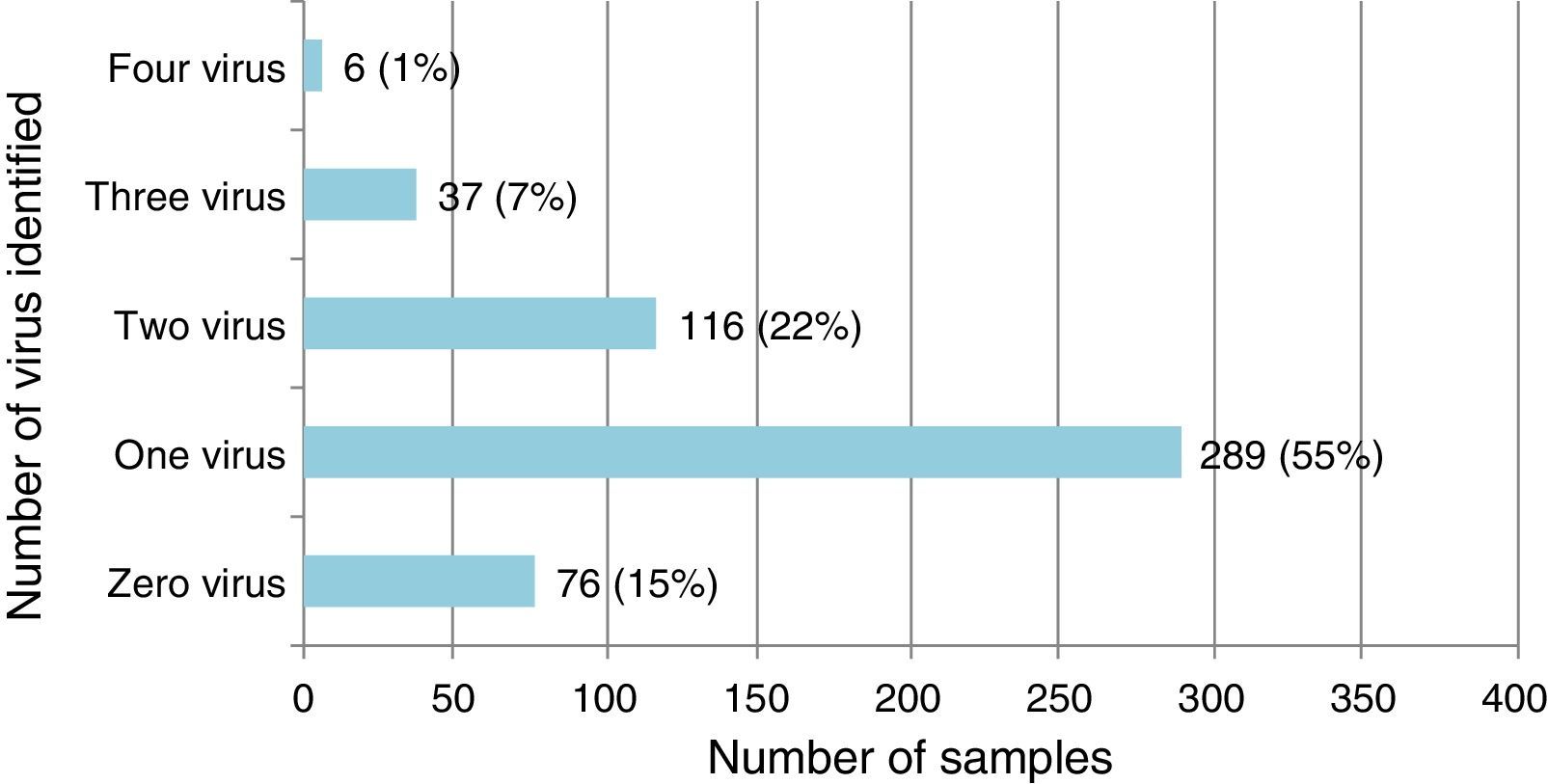
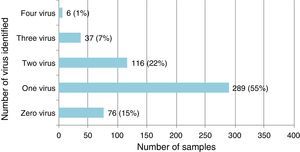
Single virus and viral coinfection among children with LRTIFrom a total of 524 respiratory specimens collected, 448 (85.5%) were positive for at least one virus (Fig. 1). RSV was the most frequently detected, followed by HRV (Table 1).
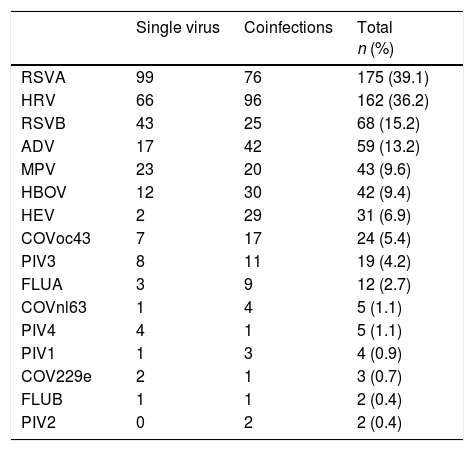
Virus identified either as single virus infection or as coinfection from the 448 positive respiratory specimens of patients below 2 years of age admitted for LRTI.
| Single virus | Coinfections | Total n (%) | |
|---|---|---|---|
| RSVA | 99 | 76 | 175 (39.1) |
| HRV | 66 | 96 | 162 (36.2) |
| RSVB | 43 | 25 | 68 (15.2) |
| ADV | 17 | 42 | 59 (13.2) |
| MPV | 23 | 20 | 43 (9.6) |
| HBOV | 12 | 30 | 42 (9.4) |
| HEV | 2 | 29 | 31 (6.9) |
| COVoc43 | 7 | 17 | 24 (5.4) |
| PIV3 | 8 | 11 | 19 (4.2) |
| FLUA | 3 | 9 | 12 (2.7) |
| COVnl63 | 1 | 4 | 5 (1.1) |
| PIV4 | 4 | 1 | 5 (1.1) |
| PIV1 | 1 | 3 | 4 (0.9) |
| COV229e | 2 | 1 | 3 (0.7) |
| FLUB | 1 | 1 | 2 (0.4) |
| PIV2 | 0 | 2 | 2 (0.4) |
Single virus infection was found in 289/448 (64.5%) of the positive cases, with RSV being the main virus detected (142 cases; 49.1%). Viral coinfection occurred in 159/448 (35.5%) cases and mainly involved RSV (101; 63.5%) and HRV (96; 60.4%) (Table 1). RSV was more frequent as single virus infection and HRV in viral coinfections, p=0.018 and 0.05 respectively.
When compared to single virus infection, viral coinfection was not more common during winter months (December to February) [103 (64.8%) vs 178 (61.6%); p=0.5].
Virus/bacteria coinfection was detected in 125/448 (27.9%) cases. Bacteria most frequently involved in coinfection were nontypeable Haemophilus influenzae in 79/125 (63%) and Streptococcus pneumoniae in 27/125 (21%).
Bronchiolitis was the most common discharge diagnosis associated with both single and viral coinfection [194 (78.9%) vs 85 (66.4%)] but pneumonia was more frequent with viral coinfection [43 (33.6%) vs 52 (21.1%); p=0.012].
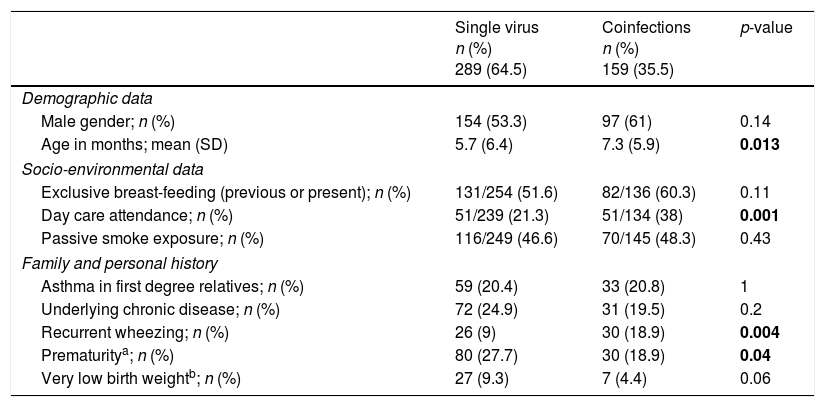
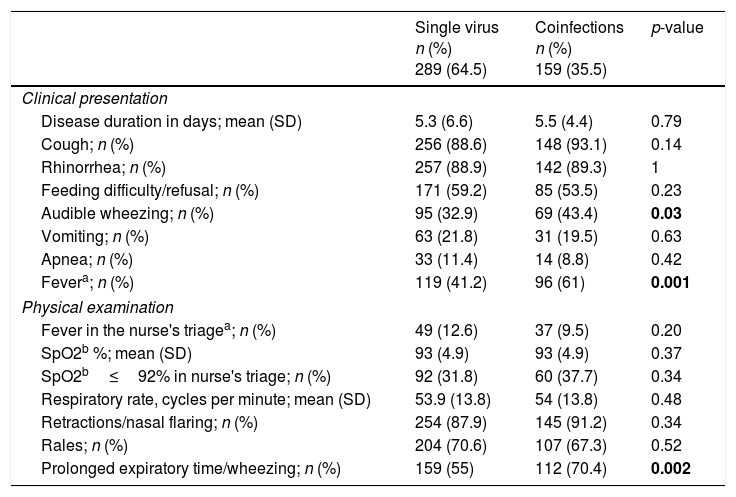
Demographic, socio-environmental and clinical characteristics of single virus and viral coinfectionsTables 2 and 3 show the demographic and socio-environmental data, personal history and clinical characteristics associated with single virus infection and viral coinfections in LRTI. Mean age on admission was significantly higher in cases of viral coinfection. Having a history of recurrent wheezing, day care attendance, audible wheezing, fever and bronchospasm on admission were more frequently associated with viral coinfection. Having been born prematurely was most commonly found in the single virus infection. All patients were discharged home or transferred to another hospital; none died.
Demographic, socio-environmental and personal history associated with single virus infections and coinfections.
| Single virus n (%) 289 (64.5) | Coinfections n (%) 159 (35.5) | p-value | |
|---|---|---|---|
| Demographic data | |||
| Male gender; n (%) | 154 (53.3) | 97 (61) | 0.14 |
| Age in months; mean (SD) | 5.7 (6.4) | 7.3 (5.9) | 0.013 |
| Socio-environmental data | |||
| Exclusive breast-feeding (previous or present); n (%) | 131/254 (51.6) | 82/136 (60.3) | 0.11 |
| Day care attendance; n (%) | 51/239 (21.3) | 51/134 (38) | 0.001 |
| Passive smoke exposure; n (%) | 116/249 (46.6) | 70/145 (48.3) | 0.43 |
| Family and personal history | |||
| Asthma in first degree relatives; n (%) | 59 (20.4) | 33 (20.8) | 1 |
| Underlying chronic disease; n (%) | 72 (24.9) | 31 (19.5) | 0.2 |
| Recurrent wheezing; n (%) | 26 (9) | 30 (18.9) | 0.004 |
| Prematuritya; n (%) | 80 (27.7) | 30 (18.9) | 0.04 |
| Very low birth weightb; n (%) | 27 (9.3) | 7 (4.4) | 0.06 |
Bold values are values with statistical significance.
Clinical presentation and physical examination findings associated with single versus viral coinfections.
| Single virus n (%) 289 (64.5) | Coinfections n (%) 159 (35.5) | p-value | |
|---|---|---|---|
| Clinical presentation | |||
| Disease duration in days; mean (SD) | 5.3 (6.6) | 5.5 (4.4) | 0.79 |
| Cough; n (%) | 256 (88.6) | 148 (93.1) | 0.14 |
| Rhinorrhea; n (%) | 257 (88.9) | 142 (89.3) | 1 |
| Feeding difficulty/refusal; n (%) | 171 (59.2) | 85 (53.5) | 0.23 |
| Audible wheezing; n (%) | 95 (32.9) | 69 (43.4) | 0.03 |
| Vomiting; n (%) | 63 (21.8) | 31 (19.5) | 0.63 |
| Apnea; n (%) | 33 (11.4) | 14 (8.8) | 0.42 |
| Fevera; n (%) | 119 (41.2) | 96 (61) | 0.001 |
| Physical examination | |||
| Fever in the nurse's triagea; n (%) | 49 (12.6) | 37 (9.5) | 0.20 |
| SpO2b %; mean (SD) | 93 (4.9) | 93 (4.9) | 0.37 |
| SpO2b≤92% in nurse's triage; n (%) | 92 (31.8) | 60 (37.7) | 0.34 |
| Respiratory rate, cycles per minute; mean (SD) | 53.9 (13.8) | 54 (13.8) | 0.48 |
| Retractions/nasal flaring; n (%) | 254 (87.9) | 145 (91.2) | 0.34 |
| Rales; n (%) | 204 (70.6) | 107 (67.3) | 0.52 |
| Prolonged expiratory time/wheezing; n (%) | 159 (55) | 112 (70.4) | 0.002 |
Bold values are values with statistical significance.
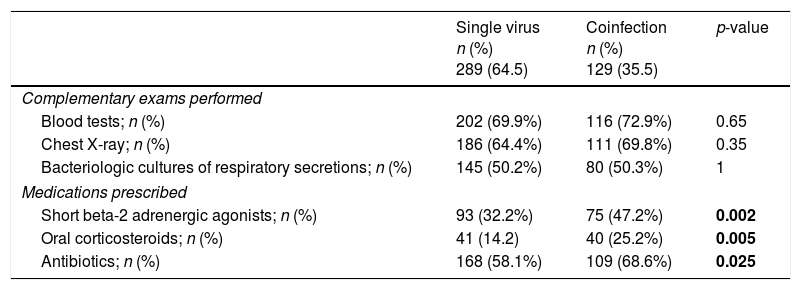
There were no significant differences between single virus infection and viral coinfection groups in what concerns complementary exams performed but medication was prescribed more regularly in the group of viral coinfection (Table 4). In both groups amoxicillin/clavulanic acid was the most frequently prescribed antibiotic (59/109 in viral coinfection and 112/168 in single virus infection).
Comparison of hospital resources’ use of single virus infection and viral coinfections.
| Single virus n (%) 289 (64.5) | Coinfection n (%) 129 (35.5) | p-value | |
|---|---|---|---|
| Complementary exams performed | |||
| Blood tests; n (%) | 202 (69.9%) | 116 (72.9%) | 0.65 |
| Chest X-ray; n (%) | 186 (64.4%) | 111 (69.8%) | 0.35 |
| Bacteriologic cultures of respiratory secretions; n (%) | 145 (50.2%) | 80 (50.3%) | 1 |
| Medications prescribed | |||
| Short beta-2 adrenergic agonists; n (%) | 93 (32.2%) | 75 (47.2%) | 0.002 |
| Oral corticosteroids; n (%) | 41 (14.2) | 40 (25.2%) | 0.005 |
| Antibiotics; n (%) | 168 (58.1%) | 109 (68.6%) | 0.025 |
Bold values are values with statistical significance.
In addition, in cases with discharge diagnosis of pneumonia, where viral coinfection was more common, short beta-2 adrenergic agonists [53 (48.6%) vs 76 (23.4%); p<0.001], oral corticosteroids [23 (21.1%) vs 17 (5.2%); p<0.001] and antibiotics [105 (96.3%) vs 165 (50.6%); p<0.001] were more often prescribed, when compared to patients with bronchiolitis.
When comparing virus/bacteria coinfection versus no bacteria isolated, blood samples [104 (84.6%) vs 215 (67.6%); p<0.001] and CXR [100 (80.6%) vs 197 (61.4%), p<0.001] were more frequently performed. Intravenous antibiotics were more commonly prescribed in the cases of virus/bacteria coinfection [116 (92.8%) vs 161 (50%); p<0.001]. For the same groups, no differences were noticed for the use of bronchodilators [40 (32%) vs 128 (39.6%); p=0.15], or corticosteroids [19 (15.2%) vs 62 (19.3%); p=0.34].
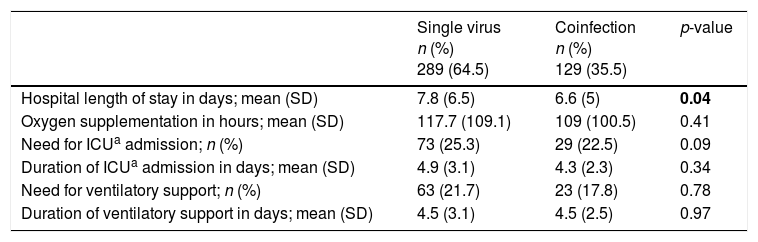
Mean LOS was slightly higher in the group of single virus infection vs viral coinfection. There were no significant differences between groups for other severity variables (Table 5).
Comparison of clinical outcomes and severity of disease of single virus infection and viral coinfections.
| Single virus n (%) 289 (64.5) | Coinfection n (%) 129 (35.5) | p-value | |
|---|---|---|---|
| Hospital length of stay in days; mean (SD) | 7.8 (6.5) | 6.6 (5) | 0.04 |
| Oxygen supplementation in hours; mean (SD) | 117.7 (109.1) | 109 (100.5) | 0.41 |
| Need for ICUa admission; n (%) | 73 (25.3) | 29 (22.5) | 0.09 |
| Duration of ICUa admission in days; mean (SD) | 4.9 (3.1) | 4.3 (2.3) | 0.34 |
| Need for ventilatory support; n (%) | 63 (21.7) | 23 (17.8) | 0.78 |
| Duration of ventilatory support in days; mean (SD) | 4.5 (3.1) | 4.5 (2.5) | 0.97 |
Bold value is value with statistical significance.
A multiple regression model showed that, after adjustment, the main variables associated with a greater LOS were prematurity [with an increase in 2.2 days (95% CI 1.0–3.5)] and having been prescribed antibiotic therapy [with an increase in 2.7 days (95% CI 1.5–3.8)]. Multivariate logistic regression model showed that, after adjustment, the main variables associated with ICU admission were the presence of acute respiratory failure/hypercarbia requiring ventilation and antibiotic prescription [OR 46.6 (95% CI 20.7–104.9) and 7.4 (95% CI 2.4–22.3), respectively]. No association between coinfection and hospital LOS or ICU admission was found with this analysis.
DiscussionIn this single-center study we found a high rate of respiratory virus detection (>85%). RSV A/B were the most frequently found viruses followed by HRV, either as single virus or coinfection, both accounting for over half of single viral identification.
The rate of viral coinfections (35.5%) was similar to that described by others using similar viral identification techniques.11,23 The most frequent viruses involved were HRV (60.4%) and RSV A (47.8%), pathogens that were also predominant in single infections, as shown in previous studies.11 ADV was the third most commonly identified in viral coinfections (26.4%). Its frequency was higher than that referred in other studies.28,29 Viruses such as ADV, HBOV, HEV, COV OC43, COV NL63, PIV1, 2 and 3 and FLUA were more frequently seen as an agent of viral coinfection than as a single agent.5,28 Nascimento et al. found similar results in 2010.23
Nevertheless, a great variability exists regarding viral coinfection rates, with results as low as 11–17.5%6,30,31 and as high as 50–60%.29,32,33 A multicenter national study7 that used immunofluorescence and PCR techniques for viral detection described a much lower coinfection rate (13.3%) in children with acute bronchiolitis. Differences in viral coinfection rates may result from the population studied (infants versus older children and different proportions and types of underlying comorbidities) or from viral diagnostic techniques used. In our study, viral coinfection was more frequent in older children, in those exposed to nursery or with individual susceptibility factors such as recurrent wheezing and also in patients with pneumoniae as the discharge diagnose. Others have reported similar results.5,6
Although there are some discrepancies between reports, we found no association between viral coinfection and clinical disease severity.5,6 In our study clinical severity was not evaluated based on a severity score, however others who have used it also found no differences.6
Asner et al.,25 in a recent meta-analysis, did not find convincing evidence that patients admitted with viral coinfection had higher risk of developing more severe disease comparing with single virus infection. However, it should be highlighted that the overall quality of evidence of the studies included applying the GRADE framework ranged from low to moderate quality of evidence. The studies of Suryadevara et al.,1 Marguet et al., Nascimento et al.,23 and De Paulis et al.11 corroborated that viral coinfection does not seem to have an impact on the severity of disease. On the other hand, Martínez-Roig et al.32 reported an inverse relationship between the number of detected viruses in the NPA, the need for oxygen supply and the hospital LOS. In the aforementioned study of Calvo et al.,6 viral coinfection appeared to be associated with a greater hospital LOS, unlike what was observed in our study.
The prescription of short-acting bronchodilators, antibiotics and systemic corticosteroids was more frequent in the viral coinfection group. We must be cautious in interpreting these data as this was probably due to children's older age and more symptomatic presentation on admission rather than the presence of viral coinfection. Some of these clinical characteristics, such as fever, were also more frequent in patients with multiple viral infections in other studies.6
Virus–bacteria coinfection, although not systematically performed, was found in 27.9% of cases, and, as previously reported, was associated with more frequent collection of blood samples, CXR and antibiotics’ prescription.5,27,34,35
There are some strengths of our study, explicitly the prospective nature of the data collection, the considerable number of respiratory samples assessed, the use of a broad molecular assay for the detection of 16 different viral respiratory pathogens and the high positivity rate. Moreover, our sample proved to be homogeneous in terms of patient's age (81.7% with less than 12 months and a median age of 4 months). Additionally, the impact of underlying comorbidities/risk factors was also addressed. Finally, an additional strength was the time span of the study including cases from all year round. Some previous studies performed seasonality-based analysis losing the characterization of some LTRI associated viruses that occur in the rest of the year.7,36
This study also has some limitations that should be acknowledged. Firstly, the fact that it was designed to describe the epidemiology of viral LRTI in hospitalized pediatric patients. As such, it included only hospitalized children in an academic medical center, so these results may not be generalizable to community attendance and cannot be extrapolated to children with less severe disease. Secondly, the viral identification through real-time PCR in respiratory secretions does not necessarily mean acute infection. It is well known that this technique is able to detect virus in up to 5% of asymptomatic patients36,37 and we should also remember that the detection of more than one virus may be due to the presence of viral fragments persisting for up to 5–6 weeks after onset of symptoms.38 Therefore, the term “co-detection” is, in fact, more precise than coinfection, especially when molecular diagnostic techniques are used. However, this limitation is difficult to overcome, since most of these viruses are difficult to grow in cell culture and, consequently, clinical interpretation of virus detection in respiratory secretions in a child with LRTI is quite challenging.21,36–38 This may be another possible reason for the absence of differences between the single infections and coinfections with regard to the clinical outcomes. Quantitative PCR and its correlation with clinical symptoms might become a useful tool to clarify the actual role of multiple infections. Finally, another important limitation is the absence of a systematic search for bacteria in the respiratory specimens. The potential influence of a concomitant bacterial infection can be a relevant issue contributing to contradictory data in studies examining the role of the viral coinfection in determining the severity of the disease. Prospective longitudinal studies including serial respiratory sampling for viruses and bacteria may lead to a better understanding of the clinical significance of polymicrobial acute LRTI.
In summary, on the basis of this prospective, single-center, multiyear data, we found that 1 in 3 children admitted for LRTI had multiple virus infection. Our results suggest that there was an association of viral coinfection with demographical, environmental and clinical characteristics on admission. Most importantly, our data challenge current thinking as we found that this does not change the short-term outcomes nor the relevance for co-hosting patients on the basis of single identification virus by other less sensitive methods.39,40
Due to the burden of LRTI in children, we highlight the need for further prospective longitudinal studies, with well-defined short-term outcomes and systematic search for viruses and bacteria in respiratory samples, in order to better understand the clinical importance of polymicrobial respiratory infections in children and adjust severity risk factors and hospital resources.
Conflicts of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Gil J, Almeida S, Constant C, Pinto S, Barreto R, Cristino JM, et al. Relevancia a corto plazo de la coinfección viral en pacientes menores de 2 años hospitalizados con infecciones de las vías respiratorias inferiores. An Pediatr (Barc). 2018;88:127–135.
Previous presentations: Congreso Extraordinario de la AEP y II Congreso Extraordinario Latinoamericano de Pediatría, Madrid, 5th to 7th of June 2014.