Pain recognition and management continues to be a challenge for health professionals in Neonatal Intensive Care Units. Many of the patients are routinely exposed to repeated painful experiences with demonstrated short- and long-term consequences. Preterm babies are a vulnerable high-risk population. Despite international recommendations, pain remains poorly assessed and managed in many Neonatal Intensive Care Units. Due to there being no general protocol, there is significant variability as regards the guidelines for the approach and treatment of pain between the different Neonatal Intensive Care Units. The objective of this article is to review and assess the general principles of pain in the initial stages of development, its recognition through the use of standardised scales. It also includes its prevention and management with the combination of pharmacological and non-pharmacological measures, as well as to establish recommendations that help alleviate pain in daily clinical practice by optimising pain and stress control in the Neonatal Intensive Care Units.
El reconocimiento del dolor y su tratamiento en las unidades de cuidados intensivos neonatales continúa siendo un desafío para los profesionales sanitarios responsables de la atención de estos niños. Las exposiciones dolorosas repetidas a las que se someten muchos de estos pacientes de manera rutinaria han demostrado presentar efectos deletéreos a corto y largo plazo. Los recién nacidos prematuros, especialmente vulnerables, suponen una población de alto riesgo. Pese a las recomendaciones internacionales, el dolor sigue siendo evaluado actualmente en muchas ocasiones de manera inconsistente, sin protocolización, siendo patente, además, entre las diferentes unidades de cuidados intensivos neonatales una variabilidad importante en cuanto a las pautas para el abordaje y tratamiento del mismo. El objetivo de este artículo es revisar y valorar los principios generales del dolor en las etapas iniciales del desarrollo, su reconocimiento mediante el uso de escalas protocolizadas, y su prevención y manejo, con la combinación de medidas farmacológicas y no farmacológicas; con el fin de establecer recomendaciones que ayuden a aliviar el dolor en la práctica clínica diaria optimizando el control del dolor y el estrés en las unidades de cuidados intensivos neonatales.
As recently as 30 years ago, when newborn infants were subjected to surgical procedures, analgesia was barely used during or after surgery. Today, we not only recognise that newborns experience pain, but its management is considered a principle of good clinical practice. In the usual hospital environment, newborns are routinely subjected to painful procedures from the first moments after birth, such as the intramuscular administration of vitamin K or the prick in the heel for the newborn metabolic screen. Newborns that require intensive care may undergo up to 10–15 painful procedures each day.1
The Neonatal Pain Control Group defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage” and stress as “an actual or perceived threat that leads to a disturbance of the dynamic equilibrium between an organism and its environment”, which are both conditions that are ever present in the neonatal intensive care unit (NICU) environment.2 The concept of comfort develops in close relationship to the definitions of “stress” and “environment”, defined by Kolcaba and DiMarco as a state of relaxation, absence of stress and physiological, emotional and social satisfaction.3
The birth process and the subsequent adaptation to extrauterine life are considered stressful experiences, and the separation of mother and infant associated to admission to the NICU has been previously described as noxious.4 In addition, due to the high complexity of the care delivered in NICUs, these units tend to have bright lights and high noise levels, with an impact on neurodevelopment that has yet to be fully established.5 Thus, setting up the appropriate environmental conditions to guarantee comfort should be a priority for all the staff involved in the care of these infants.
An increasing number of studies have been focusing on the neurologic impairment that results from overall changes in the maturation process of the brain, with particular interest on pain as a modifiable risk factor.6 This particular interest is founded, on one hand, on the increased emphasis on humane care delivery, and on the other on evidence that acute pain produces deleterious effects in the short term and recurrent painful experiences in the early stages of development have long-term deleterious effects.6,7 Consequently, the assessment, management and prevention of pain are challenges that must be addressed in the NICU setting.
The physiology of painPain has been previously described as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”. However, this definition has been heavily criticised because it excludes individuals that are not aware of their own bodies or that are unable to express it, and it is important to take into account that pain extends far beyond conventional definitions.
The existing evidence demonstrates that both term and preterm (PT) newborns have the neuroanatomical pathways required for nociception. Traditionally, the prevailing hypothesis has been that perception of pain requires the presence of certain structures connecting the thalamus to the cortex, but other authors have proposed that the spinothalamic tract is sufficient, allowing the subconscious perception of pain.8
Nociception most likely becomes possible between weeks 20 and 22 of gestation. Painful stimuli are associated with physiological, hormonal and metabolic markers as early as the week 24. It is at this point in gestation that afferent pathways become fully functional, although the autonomic and neuroendocrine self-regulating systems that modulate sensory experience are still immature (due to the incomplete development of the descending inhibitory pathways), which explains the greater vulnerability to pain of PT newborns compared to term infants.9
General measures in pain managementTo ensure appropriate pain management in newborns, it is recommended that general measures are established for the purpose in neonatal units:
- •
Systematic and routine assessment in infants of signs of pain using clinical scales and of its possible causes.
- •
Minimization or limitation of unnecessary painful stimuli and encouragement of the presence of the parents and their involvement in care delivery.
- •
Prevention and reduction of acute pain by anticipating the need for analgesia and its timely delivery, including nonpharmacological and pharmacological measures. If the latter are required, analgesic agents should be administered in a stepwise fashion based on the level of pain.
- •
Standardised protocols/guidelines for pain assessment and management.
Adequate assessment of pain is essential to guarantee a correct management and treatment of pain. Although there are international guidelines on the subject,10 there are few protocols specifying the frequency and type of pain assessments that should be performed in everyday clinical practice in Spain.11
The verbal expression of the characteristics of pain by the individual is the most suitable approach for determining the nature, location and severity of pain. Since infants are unable to speak, alternative tools had to be developed to correctly identify and assess pain in newborns. Scales to assess pain in newborns are based on the observation and recording of physiological and behavioural changes associated with pain. Their successful application requires considerable training and experience on the part of the observer. Although there are many validated scales, only 5 have exhibited an adequate level of interrater agreement (Table 1).1,12
Pain assessment scales used in newborn infants.
| Scale | Type of measurement and items | Score | Indication |
|---|---|---|---|
| Neonatal Facial Cording System Revised (NFCS-R) |
| 0–5Pain: score >3 |
|
| Premature Infant Pain Profile Revised (PIPP-R) |
| 0–18 FTNBs0–21 PTNBs <28 WGAPain: score >6Intense pain: ≥12 Requires observation of baseline behaviour before and after painful procedure |
|
| Neonatal Pain Agitation and Sedation Scale (N-PASS) |
| Pain0–11 (≤30 weeks, 1 additional point)Pain: score ≥3Sedation,0 to –10Deep: –10 to –5Mild –5 to –2 |
|
| Neonatal Infant Pain Scale (NIPS) |
| 0–7Items scored on scale of 0–1, except crying, on scale of 0–2Pain0–2 no pain/mild pain3–4 mild-moderate pain>4 severe pain |
|
| Bernese Pain Scale Neonates |
| 0–27Pain: score ≥11 |
|
HR, heart rate; FTNB, full term newborn; PTNB, preterm newborn; WGA, weeks of gestational age.
Although none of these scales can be considered ideal, as they are inadequate for assessment of prolonged pain or of newborns who are more premature or with rare clinical conditions, it is nevertheless recommended that each neonatal unit has at least one scale for the assessment of procedural pain and analgesia protocols based on the results of that scale. Due to the subjective nature of the scales and the high level of training required for their correct interpretation, new strategies are required to allow a more objective and accurate assessment of pain. Furthermore, there is evidence that environmental stress in the NICU could influence behavioural responses in newborns. Thus, cortical responses to painful stimuli in infants experiencing to greater stress levels, while having a greater amplitude, may not be reflected in their behaviour, leading to inaccurate assessments.13
There is a growing interest in the role of the cerebral cortex in the neurophysiology of pain and the transmission of noxious stimuli from nociceptors to the central nervous system, but there are still no conclusive results due to the substantial methodological heterogeneity of studies on the subject.14 Measurement of skin conductance, based on the response of the sympathetic nervous system to stress, is another of the different technologies that may be useful to measure the response to pain.15 The same objective has motivated the development of devices that allow the study of the parasympathetic response of the autonomic nervous system to a painful stimulus by measuring changes in heart rate in real time. In both cases, pilot studies have shown promising results, although the available evidence is still insufficient to warrant the widespread introduction of these methods in clinical practice.16
Pain managementNonpharmacological measuresThese measures seek to promote self-soothing and to alleviate the physiological and behavioural stress caused by acute pain during procedures such as venepuncture. They include breastfeeding, kangaroo care/skin-to-skin contact, containment, non-nutritive sucking, distraction techniques (music, smells, touch, voice…), a quiet environment, soft lighting and sucrose administration. Recently, there has been a growing interest in the presence of the parents during painful procedures.17 At other ages, the presence of the parents is a commonly used method to reduce anxiety, but in neonatology, the presence of the parents has been introduced as one among many other measures as part of a shift in the approach to neonatal care (open-door policy, kangaroo care, Newborn Individualized Developmental Care and Assessment Program [NIDCAP] etc.) and has only been analysed in relation to pain in a few observational studies, so its independent effect as a pain management measure remains unclear.
Cholecystokinin, a neuropeptide associated with analgesia, is released when newborns are exposed to the familiar odour of the mother, and therefore skin-to-skin contact with the mother may have an analgesic effect.18
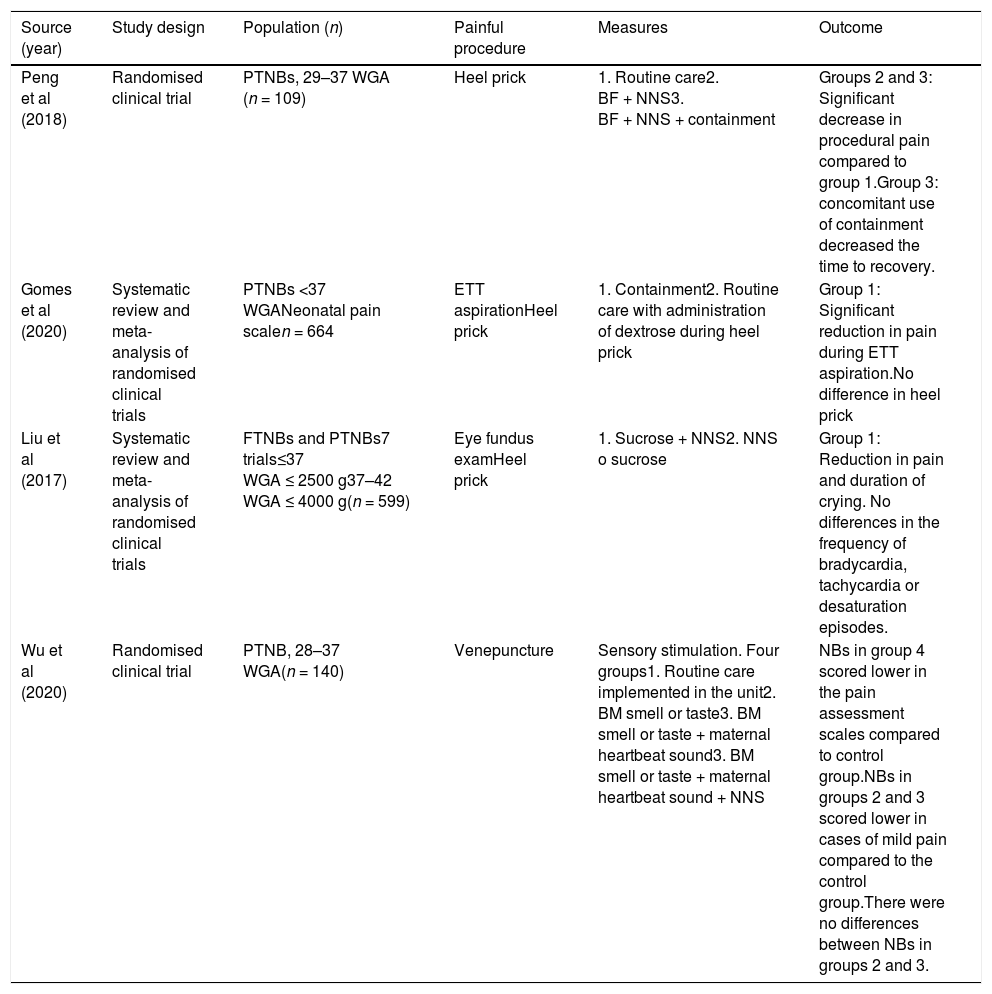
Although clinical controlled trials have been performed to evaluate each of these measures individually, due to the complexity and heterogeneity of the designs of these studies and, consequently, the low level of evidence that can be gleaned from their results, many of these measures have been underused in clinical practice for a long time. Fortunately, the number of publications in this field is growing and increasing the quality of the evidence supporting their effectiveness, especially when used in combination.19Table 2 summarises some of the clinical trials and meta-analyses on the subject published between 2017 and 2020 and the outcomes of the studied interventions.20–23
Characteristics of studies conducted in the 2017–2020 period on the combined use of nonpharmacological interventions.
| Source (year) | Study design | Population (n) | Painful procedure | Measures | Outcome |
|---|---|---|---|---|---|
| Peng et al (2018) | Randomised clinical trial | PTNBs, 29–37 WGA (n = 109) | Heel prick | 1. Routine care2. BF + NNS3. BF + NNS + containment | Groups 2 and 3: Significant decrease in procedural pain compared to group 1.Group 3: concomitant use of containment decreased the time to recovery. |
| Gomes et al (2020) | Systematic review and meta-analysis of randomised clinical trials | PTNBs <37 WGANeonatal pain scalen = 664 | ETT aspirationHeel prick | 1. Containment2. Routine care with administration of dextrose during heel prick | Group 1: Significant reduction in pain during ETT aspiration.No difference in heel prick |
| Liu et al (2017) | Systematic review and meta-analysis of randomised clinical trials | FTNBs and PTNBs7 trials≤37 WGA ≤ 2500 g37–42 WGA ≤ 4000 g(n = 599) | Eye fundus examHeel prick | 1. Sucrose + NNS2. NNS o sucrose | Group 1: Reduction in pain and duration of crying. No differences in the frequency of bradycardia, tachycardia or desaturation episodes. |
| Wu et al (2020) | Randomised clinical trial | PTNB, 28–37 WGA(n = 140) | Venepuncture | Sensory stimulation. Four groups1. Routine care implemented in the unit2. BM smell or taste3. BM smell or taste + maternal heartbeat sound3. BM smell or taste + maternal heartbeat sound + NNS | NBs in group 4 scored lower in the pain assessment scales compared to control group.NBs in groups 2 and 3 scored lower in cases of mild pain compared to the control group.There were no differences between NBs in groups 2 and 3. |
BF, breastfeeding; BM, breast milk; ETT, endotracheal tube; FT, full term; NB, newborn; NNS, non-nutritive sucking; PT, preterm; WGA, weeks of gestational age.
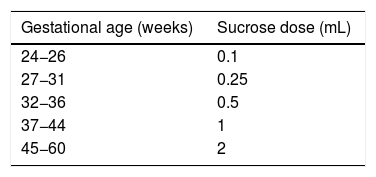
Sucrose administration is the intervention that has been researched most extensively; its use is currently approved by nearly every consensus document and clinical practice guideline.24 The underlying mechanism of action is not fully understood. It appears that it may involve the release of endogenous opioids, which suggests a potential role of dopaminergic, cholinergic or serotoninergic pathways. Sucrose is usually well tolerated, and its administration combined with non-nutritive sucking (use of a pacifier/binky) improves its effectiveness. The best outcomes have been observed with administration of sucrose in a 24% solution 2 min before exposure to the painful stimulus, with a duration of the analgesic effect of approximately 4 min. Possible short-term adverse events are rare, sporadic and temporary and include self-limiting episodes of bradycardia or desaturation. Data on the long-term effects are scarce and inconclusive. Previous research on the administration of multiple doses in the first 7–28 days post birth in PT newborns did not find differences in neurologic outcomes in the neonatal period. However, a study by Johnston et al. published in 2007 found that infants that received more than 10 doses in 24 h in the first week of life had the poorest neurodevelopmental outcomes.25 Although the optimal dosage has yet to be established, the administration of 0.2 to 0.5 mL/kg of 24% sucrose is a widely used strategy.10Table 3 presents the recommended doses of sucrose based on gestational age.26
Sucrose dose recommended based on gestational age.
| Gestational age (weeks) | Sucrose dose (mL) |
|---|---|
| 24−26 | 0.1 |
| 27−31 | 0.25 |
| 32−36 | 0.5 |
| 37−44 | 1 |
| 45−60 | 2 |
aDue to the lack of conclusive evidence on the maximum cumulative dose and its potential long-term deleterious effects, especially in preterm newborns, administration of more than 10 doses a day is discouraged.
Similarly, and with the aim of identifying an effective measure guaranteed to be safe in the long term, other “sweet solutions” must be investigated as alternatives to sucrose. In this regard, we ought to highlight the analgesic effect of the administration of maternal milk. While reviews published to date have not clearly proven the superiority of human milk over sucrose, with similar outcomes in terms of efficacy, harmlessness and multiple other benefits, maternal milk should be considered the first choice. In a recent study that assessed the response of the cerebral cortex to the implementation of different nonpharmacological analgesia strategies, Bembich et al.27 observed greater activation of the somatosensory and primary motor cortex with the administration of maternal milk (previously extracted or directly from the breast) compared to administration of sucrose, paving the way for future research.
Pharmacological measuresPharmacological analgesia is indicated for moderate to severe pain in combination with nonpharmacological measures to optimise outcomes. Analgesics are drugs that are mainly metabolised by the liver and eliminated through the kidneys, with substantial variability between individuals in their distribution and clearance that is even more marked in PT infants.
There are few studies on the safety of these drugs in newborn infants, particularly those born preterm. In animal models, exposure of the immature central nervous system to volatile anaesthetics, benzodiazepines, propofol, barbiturates and ketamine was associated with neurotoxicity. The agonist effect on gamma-aminobutyric acid receptors and antagonist effect on N-methyl-d-aspartate receptors of systemic anaesthetics seem to be responsible for the neurodegenerative effect through generalised neuronal apoptosis and impaired synaptogenesis, which in the long term give rise to cognitive and behavioural deficits.28 However, the few studies that have assessed neurotoxicity in newborns have not yielded conclusive results.29 In agreement with the findings of animal models, which have shown greater neurodevelopmental impairment in association with a combination of several drugs and prolonged use, neonatal exposure to a single anaesthetic drug and for a short period of time seems safe in healthy individuals,30,31 although prospective studies are required to assess the impact of repeated doses and prolonged treatment with one or more anaesthetic agents and in the most vulnerable patients, such as PT infants.
The potential neuroprotective effect of dexmedetomidine is the reason why this drug, which as sedative and analgesic effects, has been considered as a possible option for newborns; in foetal and neonatal animal models, its use has not been found to induce neural apoptosis and it has even been associated with a decrease in the neurotoxicity caused by volatile anaesthetics (isoflurane), propofol and ketamine when administered in combination.32 While clinical trials in newborns need to be performed to assess its neuroprotective effects, the use of dexmedetomidine to date in this population, including PT infants delivered before 28 weeks’ gestation, has been effective and well tolerated in the absence of clinically significant adverse events.33
Although in the past it was widely used in newborns, the neurotoxic effects associated with the administration of midazolam in this population (altered level of consciousness, absence of visual tracking, hypertonia, hypotonia, dyskinesis, myoclonus, apnoea, bradycardia), in part secondary to a transient decrease in cerebral perfusion and blood pressure, have led to the current recommendation against its use in PT newborns delivered before 32 weeks’ gestational age. In a Cochrane review of the only 3 randomised controlled trials that compared continuous intravenous infusion of midazolam (20–60 µg/kg/h) with placebo, one of the trials had found a statistically significant increase in the incidence of neurologic adverse events: death, severe intraventricular haemorrhage (grade III-IV) and periventricular leukomalacia.34
The neurotoxic effect of opioids, which results from their modulatory effect on neuronal proliferation and apoptosis, has also been described in experimental studies. Similarly, although studies that have assessed the impact of neonatal exposure to opioids on neurodevelopment have yielded heterogeneous results, they, too, suggest that exposure to high doses and prolonged treatment may be associated with adverse outcomes, especially in PT newborns, while the use of these drugs at low doses seemed safe.
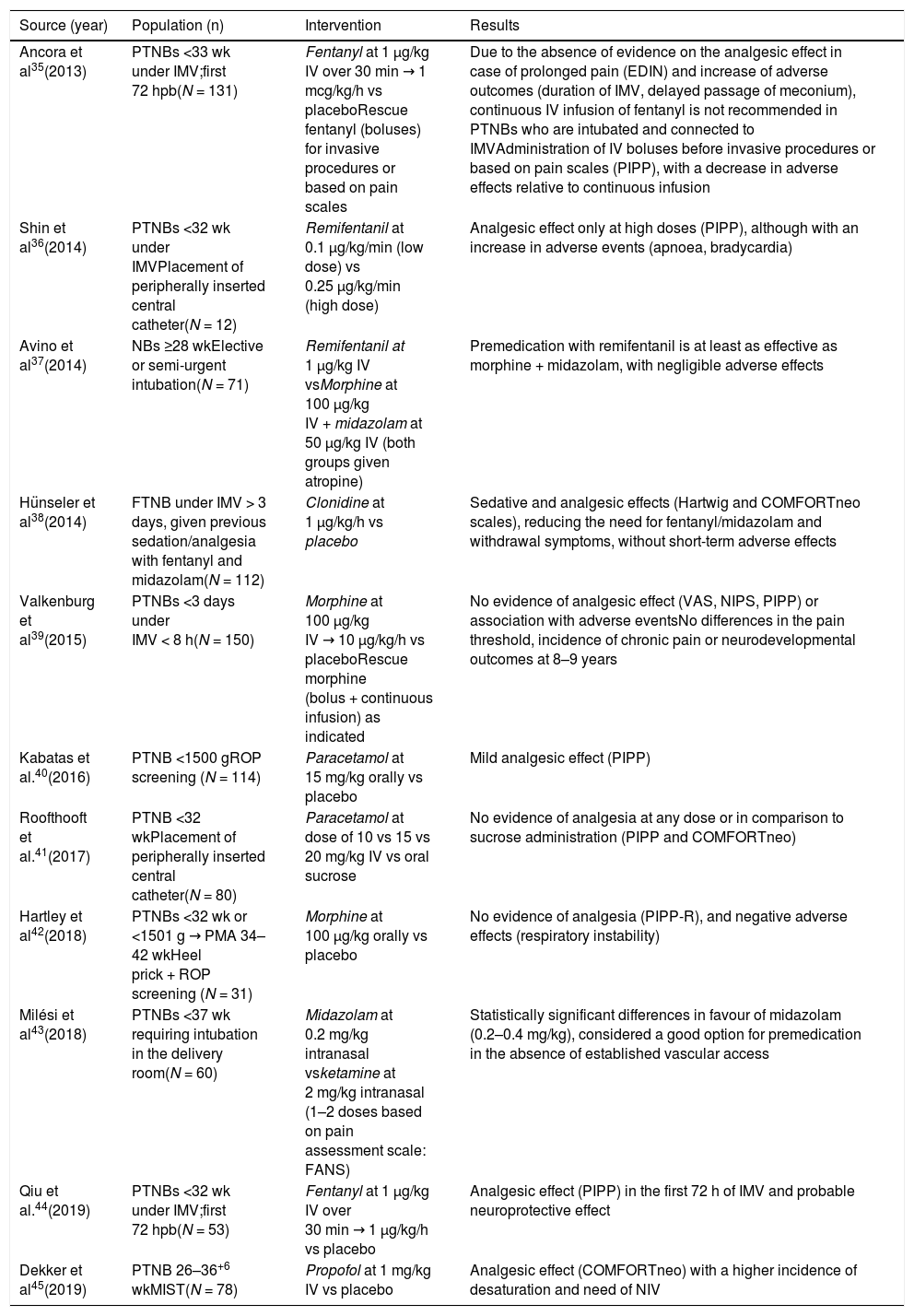
The current evidence evinces the need to continue to assess the efficacy, safety and titration of drugs available for use in newborns, as well as to promote their administration in combination with nonpharmacological measures with the aim of using the minimum effective dose and minimise adverse events. Table 4 presents the clinical trials of drugs used for pain relief in newborns conducted in the 2014–2020 period.35–45
Clinical trials of drugs used for pain relief in newborns.
| Source (year) | Population (n) | Intervention | Results |
|---|---|---|---|
| Ancora et al35(2013) | PTNBs <33 wk under IMV;first 72 hpb(N = 131) | Fentanyl at 1 µg/kg IV over 30 min → 1 mcg/kg/h vs placeboRescue fentanyl (boluses) for invasive procedures or based on pain scales | Due to the absence of evidence on the analgesic effect in case of prolonged pain (EDIN) and increase of adverse outcomes (duration of IMV, delayed passage of meconium), continuous IV infusion of fentanyl is not recommended in PTNBs who are intubated and connected to IMVAdministration of IV boluses before invasive procedures or based on pain scales (PIPP), with a decrease in adverse effects relative to continuous infusion |
| Shin et al36(2014) | PTNBs <32 wk under IMVPlacement of peripherally inserted central catheter(N = 12) | Remifentanil at 0.1 µg/kg/min (low dose) vs 0.25 µg/kg/min (high dose) | Analgesic effect only at high doses (PIPP), although with an increase in adverse events (apnoea, bradycardia) |
| Avino et al37(2014) | NBs ≥28 wkElective or semi-urgent intubation(N = 71) | Remifentanil at 1 µg/kg IV vsMorphine at 100 µg/kg IV + midazolam at 50 µg/kg IV (both groups given atropine) | Premedication with remifentanil is at least as effective as morphine + midazolam, with negligible adverse effects |
| Hünseler et al38(2014) | FTNB under IMV > 3 days, given previous sedation/analgesia with fentanyl and midazolam(N = 112) | Clonidine at 1 µg/kg/h vs placebo | Sedative and analgesic effects (Hartwig and COMFORTneo scales), reducing the need for fentanyl/midazolam and withdrawal symptoms, without short-term adverse effects |
| Valkenburg et al39(2015) | PTNBs <3 days under IMV < 8 h(N = 150) | Morphine at 100 µg/kg IV → 10 µg/kg/h vs placeboRescue morphine (bolus + continuous infusion) as indicated | No evidence of analgesic effect (VAS, NIPS, PIPP) or association with adverse eventsNo differences in the pain threshold, incidence of chronic pain or neurodevelopmental outcomes at 8–9 years |
| Kabatas et al.40(2016) | PTNB <1500 gROP screening (N = 114) | Paracetamol at 15 mg/kg orally vs placebo | Mild analgesic effect (PIPP) |
| Roofthooft et al.41(2017) | PTNB <32 wkPlacement of peripherally inserted central catheter(N = 80) | Paracetamol at dose of 10 vs 15 vs 20 mg/kg IV vs oral sucrose | No evidence of analgesia at any dose or in comparison to sucrose administration (PIPP and COMFORTneo) |
| Hartley et al42(2018) | PTNBs <32 wk or <1501 g → PMA 34–42 wkHeel prick + ROP screening (N = 31) | Morphine at 100 µg/kg orally vs placebo | No evidence of analgesia (PIPP-R), and negative adverse effects (respiratory instability) |
| Milési et al43(2018) | PTNBs <37 wk requiring intubation in the delivery room(N = 60) | Midazolam at 0.2 mg/kg intranasal vsketamine at 2 mg/kg intranasal (1–2 doses based on pain assessment scale: FANS) | Statistically significant differences in favour of midazolam (0.2–0.4 mg/kg), considered a good option for premedication in the absence of established vascular access |
| Qiu et al.44(2019) | PTNBs <32 wk under IMV;first 72 hpb(N = 53) | Fentanyl at 1 µg/kg IV over 30 min → 1 µg/kg/h vs placebo | Analgesic effect (PIPP) in the first 72 h of IMV and probable neuroprotective effect |
| Dekker et al45(2019) | PTNB 26–36+6 wkMIST(N = 78) | Propofol at 1 mg/kg IV vs placebo | Analgesic effect (COMFORTneo) with a higher incidence of desaturation and need of NIV |
FT, full term; hpb, hours post birth; IMV, invasive mechanical ventilation; IV, intravenous; NB, newborn; NIV, non-invasive ventilation; PMA, postmenstrual age; PT, preterm; ROP, retinopathy of prematurity; wk, weeks.
Pain assessment scales: EDIN, Echelle Douleur Inconfort Nouveau-N; FANS, ‘Faceless’ Acute Neonatal pain Scale; NIPS, Neonatal Infant Pain Profile; PIPP, Premature Infant Pain Profile; PIPP-R, Premature Infant Pain Profile-Revised; VAS, Visual Analog Scale.
There is substantial variability in the use of pharmacological measures for pain management between NICUs, both in Spain and at the international level,46 and there are no established protocols or consensus guidelines on the subject. Although in recent years measures for the prevention and treatment of procedural pain have been widely implemented in neonatal units, the use of sedation or analgesia in subacute or chronic care interventions, such as invasive mechanical ventilation, remains controversial.35
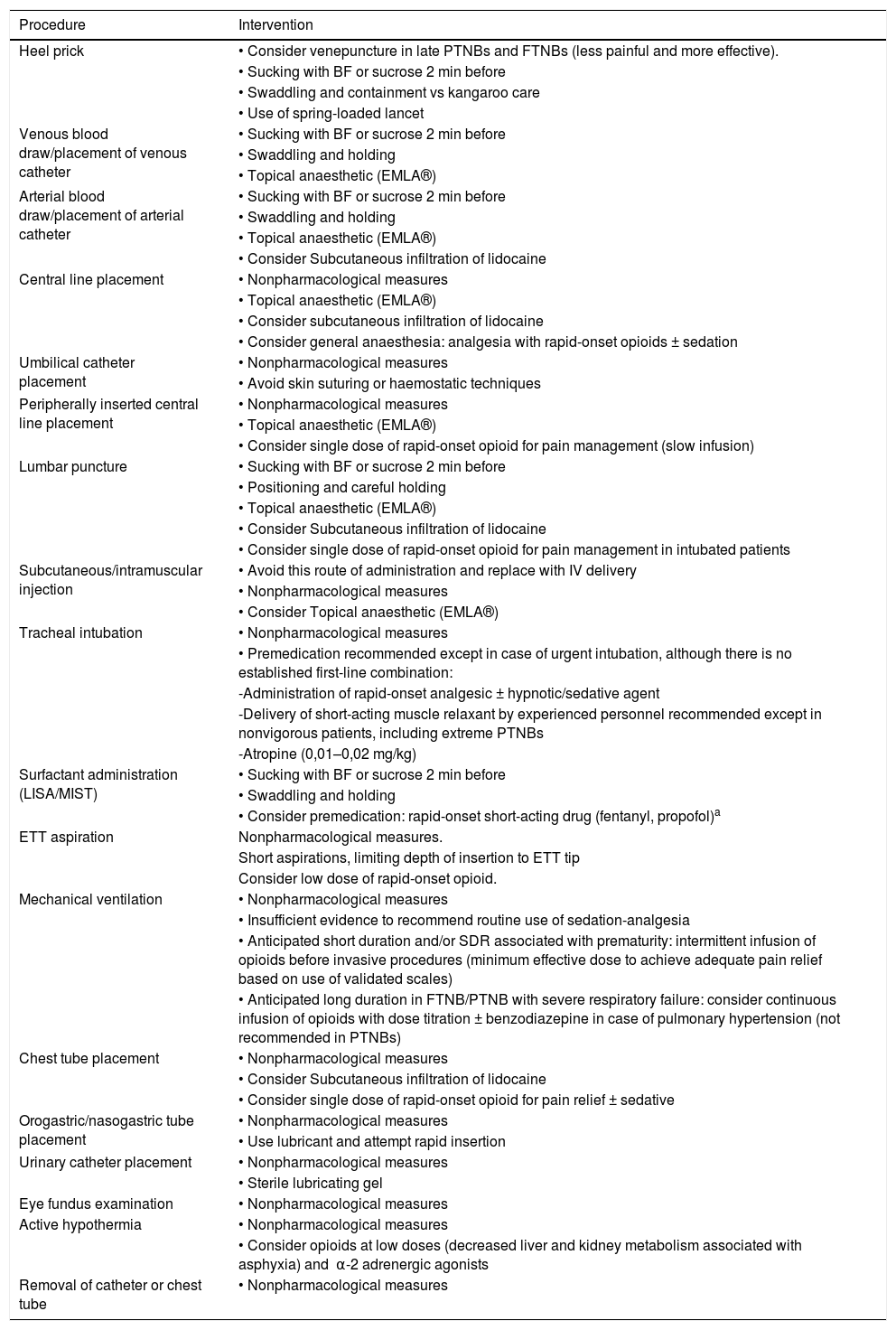
Table 5 provides recommendations on the pharmacological and nonpharmacological pain relief measures to use in the most frequent situations and procedures in neonatal clinical practice.47,48
Sedation and analgesia in specific situations/procedures.
| Procedure | Intervention |
|---|---|
| Heel prick | • Consider venepuncture in late PTNBs and FTNBs (less painful and more effective). |
| • Sucking with BF or sucrose 2 min before | |
| • Swaddling and containment vs kangaroo care | |
| • Use of spring-loaded lancet | |
| Venous blood draw/placement of venous catheter | • Sucking with BF or sucrose 2 min before |
| • Swaddling and holding | |
| • Topical anaesthetic (EMLA®) | |
| Arterial blood draw/placement of arterial catheter | • Sucking with BF or sucrose 2 min before |
| • Swaddling and holding | |
| • Topical anaesthetic (EMLA®) | |
| • Consider Subcutaneous infiltration of lidocaine | |
| Central line placement | • Nonpharmacological measures |
| • Topical anaesthetic (EMLA®) | |
| • Consider subcutaneous infiltration of lidocaine | |
| • Consider general anaesthesia: analgesia with rapid-onset opioids ± sedation | |
| Umbilical catheter placement | • Nonpharmacological measures |
| • Avoid skin suturing or haemostatic techniques | |
| Peripherally inserted central line placement | • Nonpharmacological measures |
| • Topical anaesthetic (EMLA®) | |
| • Consider single dose of rapid-onset opioid for pain management (slow infusion) | |
| Lumbar puncture | • Sucking with BF or sucrose 2 min before |
| • Positioning and careful holding | |
| • Topical anaesthetic (EMLA®) | |
| • Consider Subcutaneous infiltration of lidocaine | |
| • Consider single dose of rapid-onset opioid for pain management in intubated patients | |
| Subcutaneous/intramuscular injection | • Avoid this route of administration and replace with IV delivery |
| • Nonpharmacological measures | |
| • Consider Topical anaesthetic (EMLA®) | |
| Tracheal intubation | • Nonpharmacological measures |
| • Premedication recommended except in case of urgent intubation, although there is no established first-line combination: | |
| -Administration of rapid-onset analgesic ± hypnotic/sedative agent | |
| -Delivery of short-acting muscle relaxant by experienced personnel recommended except in nonvigorous patients, including extreme PTNBs | |
| -Atropine (0,01–0,02 mg/kg) | |
| Surfactant administration (LISA/MIST) | • Sucking with BF or sucrose 2 min before |
| • Swaddling and holding | |
| • Consider premedication: rapid-onset short-acting drug (fentanyl, propofol)a | |
| ETT aspiration | Nonpharmacological measures. |
| Short aspirations, limiting depth of insertion to ETT tip | |
| Consider low dose of rapid-onset opioid. | |
| Mechanical ventilation | • Nonpharmacological measures |
| • Insufficient evidence to recommend routine use of sedation-analgesia | |
| • Anticipated short duration and/or SDR associated with prematurity: intermittent infusion of opioids before invasive procedures (minimum effective dose to achieve adequate pain relief based on use of validated scales) | |
| • Anticipated long duration in FTNB/PTNB with severe respiratory failure: consider continuous infusion of opioids with dose titration ± benzodiazepine in case of pulmonary hypertension (not recommended in PTNBs) | |
| Chest tube placement | • Nonpharmacological measures |
| • Consider Subcutaneous infiltration of lidocaine | |
| • Consider single dose of rapid-onset opioid for pain relief ± sedative | |
| Orogastric/nasogastric tube placement | • Nonpharmacological measures |
| • Use lubricant and attempt rapid insertion | |
| Urinary catheter placement | • Nonpharmacological measures |
| • Sterile lubricating gel | |
| Eye fundus examination | • Nonpharmacological measures |
| Active hypothermia | • Nonpharmacological measures |
| • Consider opioids at low doses (decreased liver and kidney metabolism associated with asphyxia) and α-2 adrenergic agonists | |
| Removal of catheter or chest tube | • Nonpharmacological measures |
BF, breastfeeding; EMLA®, lidocaine + prilocaine; ETT, endotracheal tube; FTNB, full term newborn; LISA, less invasive surfactant administration; MIST, minimally invasive surfactant therapy; PTNB, preterm newborn; RDS, respiratory distress syndrome.
EMLA®: up to 1 g and 10 cm2 over 1 h, single dose in 24 h. Although the summary of product characteristics does not recommend its use in PTNBs (<37 weeks) due to the risk of methaemoglobinaemia, the only clinical trial that assessed the safety of EMLA® versus placebo in PTNB delivered at 30–36 weeks’ gestation did not find clinically significant levels of methaemoglobin (>5%) in any of the patients.47.
At present, there is no consensus on premedication due to the absence of a drug with a predictable pharmacokinetic profile that allows maintenance of spontaneous breathing, thereby preventing certain short- and long-term adverse effects, although new studies are required to investigate the ideal sedation and analgesia scheme to achieve an adequate balance between the maintenance spontaneous breathing and the treatment of pain associated with surfactant administration.48.
The assessment of pain needs to be standardised and become a routine practice in neonatal units to optimise pain relief, if pain cannot be avoided, through the combination of pharmacological and nonpharmacological measures, titrating drugs to the minimum effective dose and minimising potential adverse events. Randomised controlled trials need to be developed to assess the short- and long-term impact of exposure to the sedatives and analgesics that are currently available and to establish protocols for the management of stress and pain in the neonatal population. Research in neuroprotection could facilitate adequate pain management in these patients while protecting the developing brain.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Espinosa Fernández MG, González-Pacheco N, Sánchez-Redondo MD, Cernada M, Martín A, Pérez-Muñuzuri A, et al. Sedoanalgesia en las unidades neonatales. An Pediatr (Barc). 2021;95:126.