Corticosteroids have had a central role in the treatment of nephrotic syndrome. The management of these patients who become dependent to steroids is complex, involving different immunosuppressive drugs patterns. The monoclonal antibody anti CD20, Rituximab, is likely to have beneficial effects in cases of steroid-dependent nephrotic syndrome patients with no easy resolution, even when we cannot make a statement about the specific role in the impact. We bring our personal experience in pediatric patients treated with this medication during the last years, to provide a thorough overview and useful information about the role of Rituximab in this pathology.
MethodsRetrospective study in patients with steroid-dependent idiopathic nephrotic syndrome controlled in the division of Pediatric Nephrology of a Spanish tertiary hospital in those patients who had received at least one treatment cycle of Rituximab, at any moment along the evolution of the disease.
ResultsThe study involved 8 patients. All of them previously received immunosuppressive therapy. The Rituximab were administered as an intravenous infusion, in a dose of 375 mg/m2, and all doses were administered in a period during which the disease was in remission. The depletion of lymphocytes B (CD19, 0%) were confirmed after the first dose of Rituximab except for one, with a lymphocyte count of 1%. The period of depletion lasts 10,3 months (median; range 6,5–16 months), and only one of the patients registered a relapse of the disease in this period. A reduction of relapses suffered by patients has been shown after the treatment began (3,6 relapses/year in the previous year to the start of the treatment versus 0,1 relapses/year during the first year post-rituximab). The relapse-free survival in the first year reached 83,3% in patients who suffered more than one relapse (75% of patients), and without a relapse after the treatment began in 2 cases. One or more drugs could be removed in 87,5% of patients after the first cycle of rituximab. After the rituximab treatment, we reached a 96,5% decrease in the corticosteroids doses administered (28,5 mg/m2/day during the 3 months pre-treatment versus 1 mg/m2/day in the last 3 months of patient monitoring). Not a significant observed adverse effect attributed to the drug after the post-rituximab monitoring period (median 46,5 months, range 5–97 months).
ConclusionThe favorable results reported after rituximab treatment in our patients seems to confirm the effectiveness of this drug in the steroid-dependent nephrotic syndrome, making that therapeutic option into consideration and legitimating the use of the drug in complex cases involving pediatric patients. Even so, it seems recommendable to design pertinent studies to clarify, among others, the optimum regimen of the treatment (dose, interval and cycles), clinical repercussion and potential adverse effects in long terms.
La corticoterapia continúa siendo la piedra angular en el tratamiento del síndrome nefrótico. El manejo de los pacientes que desarrollan dependencia a esteroides es complejo, implicando distintas pautas de fármacos inmunosupresores. El rituximab, anticuerpo monoclonal anti-CD20, parece tener efectos beneficiosos en pacientes con síndrome nefrótico córtico-dependiente de difícil manejo clínico, si bien aún no está bien definido su papel en esta entidad. Con el fin de aportar información útil sobre el papel del rituximab en esta patología, presentamos nuestra experiencia personal en pacientes pediátricos tratados con este fármaco en los últimos años.
Materiales y métodosEstudio retrospectivo en pacientes con síndrome nefrótico idiopático cortico-dependiente controlados en la Sección de Nefrología Pediátrica de un hospital terciario español y que habían recibido, al menos, un ciclo de tratamiento con rituximab durante cualquier momento de la evolución de la enfermedad.
ResultadosSe incluyeron en el estudio 8 pacientes. Todos habían recibido terapia inmunosupresora previamente. El rituximab se administró por vía intravenosa a una dosis de 375 mg/m2, y todas las dosis se administraron en situación de remisión de la enfermedad. Se confirmó la depleción completa de linfocitos B (CD19, 0%) tras la primera dosis de rituximab en todos excepto en uno, en el que el recuento fue del 1%. El período de depleción fue de 10,3 meses (mediana; rango 6,5–16 meses), y solamente en uno de los pacientes se registró una recaída de la enfermedad durante este período. Tras el inicio del tratamiento, se observó una reducción del número de recaídas (3,6 recaídas/año en el año previo al inicio del tratamiento versus 0,1 recaídas/año durante el primer año post-rituximab). La supervivencia libre de recaída en el primer año fue del 83,3% en los pacientes con más de una recaída (75% de los pacientes), sin existir recaídas tras el inicio del tratamiento en 2 pacientes. En un 87,5% de los pacientes se pudieron retirar uno o más fármacos tras el primer ciclo de rituximab. Tras el tratamiento con rituximab, se consiguió una reducción del 96,5% de las dosis de corticoides administrados (28,5 mg/m2/día en los 3 meses pre-tratamiento versus 1 mg/m2/día en los 3 últimos meses de seguimiento en los pacientes tratados). Tras el periodo de seguimiento post-rituximab (mediana 46,5 meses, rango 5–97 meses), no se observaron efectos adversos significativos atribuibles al fármaco.
ConclusionesLos resultados favorables reportados tras el tratamiento con rituximab en nuestros pacientes parecen confirmar la eficacia del fármaco en el síndrome nefrótico córtico-dependiente y lo hacen considerar como una opción terapéutica válida en casos de difícil manejo clínico en la edad pediátrica. Aun así, parece recomendable diseñar estudios apropiados que traten de aclarar, entre otros, el régimen óptimo de tratamiento (dosis, intervalo y número de ciclos), repercusión clínica y potenciales efectos adversos a largo plazo.
Nephrotic syndrome (NS) is the most frequent chronic glomerular disease in the paediatric population. It is defined as glomerular disease with urinary protein excretion greater than 40 mg/m2/h, low serum albumin levels under 2.5 g/dL and oedema. The incidence of NS is of 2–7 new cases per 100 000 children per year, with a higher prevalence (15 cases per 100 000 children) on account of the protracted course of disease. It is more frequent in boys (2:1) and usually has onset between ages 2 and 8 years, with an incidence peak between 3 and 5 years. Idiopathic NS amounts to 90% of cases of NS in children, and minimal change disease (MCD) is the most frequent histological type, followed by focal segmental glomerulosclerosis (FSGS).
Steroid therapy is the cornerstone of NS treatment. Most children respond to this approach, although 20% develop resistance to steroids at some point in the course of disease. About 50% of patients with steroid-sensitive NS have frequent relapses (more than 2 relapses in the first 6 months or more than 3 at any time in the course of disease), of who approximately 50%–60% experience relapses during steroid therapy or soon after achieving remission, which is known as steroid-dependent NS.
Immunosuppressive agents, such as cyclophosphamide, chlorambucil, levamisole, mycophenolate mofetil, cyclosporin A and/or tacrolimus may be used to attempt to reduce or eliminate steroid therapy, although there is no consensus on the selection, duration and sequence of these second-line drugs. In 2004, evidence was first published suggesting that rituximab (RTX), a chimeric anti-CD20 monoclonal antibody that inhibits CD20+ B cell proliferation and differentiation, could be beneficial for treatment of NS. The mechanisms of action of RTX in NS are not well understood, but in addition to B cells, RTX may also act on targets found in T cells, inflammatory cytokines and even podocytes.1
Since 2004, different studies have produced promising evidence on the role of this antibody in patients with difficult-to-treat NS, although its specific indications, appropriate dosage and schedule or long-term adverse effects have yet to be established. We present our experience with patients treated with RTX in recent years with the aim of contributing information that may help define the role of RTX in the management of steroid-dependent idiopathic NS.
Material and methodsWe conducted a retrospective study in patients with steroid-dependent idiopathic NS managed in the Section of Paediatric Nephrology of a tertiary care hospital in Spain that received at least one cycle of RTX at any point in the course of disease.
Rituximab treatment protocolBefore infusion of each cycle of RTX, we performed a complete blood count, blood chemistry panel, serologic tests for detection of hepatitis B and C, lymphocyte subset panel and immunoglobulin levels, and a chest radiograph was performed only before the first cycle. Adhering to current recommendations,2,3 all doses of RTX were given when the patient was in remission induced by conventional steroid therapy, so that at the time of RTX administration all patients were receiving prednisone at a dose of 40 mg/m2/48 h. All other immunosuppressant drugs were suspended before the administration of RTX.
Patients received RTX in the day hospital (as intravenous solution in physiological saline at a concentration of 1 mg/mL) at a dose of 375 mg/m2. All patients were premedicated 30−60 min before administration of RTX with paracetamol (15 mg/kg) and dexchlorpheniramine (0.1 mg/kg), delivered orally, and a steroid (hydrocortisone at 4 mg/kg or methylprednisolone at 1–1.5 mg/kg), delivered intravenously, to minimise potential adverse effects. The first dose of RTX was delivered over 4–6 h, starting at a rate of 25 mL/h that increased progressively to 150–200 mL/h if the patient exhibited adequate tolerance. Subsequent doses were administered at a faster rate over a period of approximately 3 h.
A week after the first dose of RTX, after verifying B cell depletion, patients received prophylaxis against Pneumocystis with trimethoprim-sulfamethoxazole and steroid therapy was tapered off (usually by 10 mg/m2 every 2 weeks) until is complete elimination. The earliest patients in the series also received elective treatment with mycophenolate mofetil starting 3 months after the initial RTX dose.
We assessed the response to RTX through trimestral clinical assessments and a complete blood count with measurement of B cell counts and immunoglobulin levels at similar intervals until normalization of lymphocyte subsets. If hypogammaglobulinemia persisted, immunoglobulin levels continued to be measured in every subsequent follow-up evaluation.
The approach to relapse in every patient included in the study was to induce remission with steroid therapy alone and schedule a new RTX cycle once remission was achieved.
Clinical variables under studyWe collected the following data from health records: (1) patient and NS characteristics (sex, age at diagnosis and course of NS, histology, renal function, immunosuppressant treatment received from onset, age at initiation of RTX); (2) baseline condition of the patient at initiation of RTX (relapses in the past year, immunosuppressant drug dosage in the past 3 months); (3) characteristics of treatment with RTX (number of doses and cycles given, antibiotherapy during treatment, immunosuppressant drugs and other drugs given during treatment, adverse events during infusion, analysis of B cell depletion period); and (4) response to RTX estimated based on the periods of remission or relapse, need of subsequent doses of RTX, steroid therapy and other immunosuppressant agents.
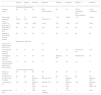
ResultsBaseline characteristics of the patientsThe study included 8 patients (5 female, 3 male), with a median age at onset 2.7 years (range, 2.0–4.9) and a median duration of NS at initiation of RTX of 9.6 years (range, 2.9–14.8). All patients had a diagnosis of steroid-dependent NS. Five had undergone a renal biopsy prior to treatment with RTX: 3 had histological features compatible with minimal change disease, 1 with focal segmental glomerulosclerosis and 1 with IgM nephropathy. All patients had a glomerular filtration rate in the normal range for age at the time of initiation of RTX (Table 1).
Description of patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Patient description | ||||||||
| Sex | Male | Female | Female | Female | Male | Female | Female | Male |
| Age at onset (years) | 2.2 | 3.1 | 2 | 2.4 | 2.3 | 4.9 | 3.2 | 2.9 |
| Renal biopsy | Minimal change | Not performed | Minimal change | Minimal change | Not performed | FSGS | Not performed | IgM nephropathy |
| Year of RTX initiation | 2015 | 2017 | 2013 | 2012 | 2013 | 2017 | 2018 | 2010 |
| Age at RTX initiation (years) | 17 | 12.9 | 5 | 9.1 | 15.5 | 9.2 | 13 | 14.3 |
| Duration of SN (years) | 14.8 | 9.7 | 2.9 | 7.6 | 13.2 | 4.2 | 9.5 | 11.4 |
| CrCl (mL/min/1.73 m2) | 168.2 | 183.3 | 175.9 | 129.5 | 87.8 | 126.3 | 159.3 | 168.3 |
| Condition prior to RTX initiation | ||||||||
| Recurrences in preceding year | 2 | 2 | 5 | 6 | 6 | 2 | 4 | 2 |
| IS before RTX | Steroids, CPA, MPM, FK, CP | Steroids, CPA, MPM | Steroids, CPA, MPM, CP, levamisole | Steroids, CPA, MPM, FK, CP | Steroids, CPA, MPM | Steroids, CPA | Steroids, CPA, MPM | Steroids, CPA, MPM, CP |
| IS dose 3 months preceding RTX* | Steroids: 8.88 | Steroids: 9.80 | Steroids: 43.38 | Steroids: 34.50 | Steroids: 20.60 | Steroids: 18.47 | Steroids: 38.80 | Steroids: 53.27 |
| FK: 0.16 | MPM: 987.00 | CPA: 4.57 | FK: 0.05 | MPM: 480.18 | CPA: 4.57 | MPM:1036.00 | MPM: 980.00 | |
CP, cyclophosphamide; CPA, cyclosporin A; CrCl, creatinine clearance; FK, tacrolimus; FSGS, focal segmental glomerulosclerosis; IS, immunosuppressant drugs; MPM, mycophenolate mofetil; RTX, rituximab.
The median age at the initial dose of RTX was 12.9 years (range, 5.0–17.0). Before the first cycle of RTX, every patient had been treated with steroids and different immunosuppressant drugs, including, in order of decreasing frequency, ciclosporin A (100%), mycophenolate mofetil (87.5%), cyclophosphamide (50%), tacrolimus (25%) and levamisole (12.5%). Patients had experienced a mean of 3.6 relapses in the year preceding initiation of RTX, and each relapse was managed with conventional steroid therapy. In addition to steroid therapy, all patients received another immunosuppressant drug in the 3 months preceding initiation of RTX: 4 received mycophenolate mofetil (mean dose, 870.8 mg/m2/day), 2 ciclosporin A (mean dose, 3.6 mg/kg/day) and 2 tacrolimus (mean dose, 0.1 mg/kg/day).
Treatment with RTX and clinical responseThe initial regimen consisted of a cycle of 2 weekly infusions of RTX in 6 patients, a single infusion in 1 patient and 4 weekly infusions in 1 patient. After the initial cycle, 2 patients did not require further doses of RTX, while 6 did require additional cycles (range, 2–7 cycles) to stay in remission (Table 2).
Characteristics of treatment.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Treatment characteristics | ||||||||
| Adverse events | No | No | No | Mild bronchospasm | No | No | Facial exanthema, mild bronchospasm | Facial exanthema |
| RTX cycles (doses per cycle) | 3 (2/2/2) | 2 (2/1) | 2 (2/2) | 7 (2/2/2/2/1/1/1) | 1 (2) | 2 (2/2) | 1 (1) | 2 (4/3) |
| Total RTX dose (mg/m²) | 2.250 | 1.125 | 1.500 | 4.125 | 750 | 1.500 | 375 | 2.625 |
| MPM after 3 months of RTX | No | No | Yes | Yes | Yes | No | No | Yes |
| Additional RTX doses without relapse | No | No | No | No | No | No | No | No |
| Response to treatment | ||||||||
| B cell count after first dose of RTX (%) | 0.2 | 1 | ||||||
| Time to B cell recovery (months) | 13 | 8 | 12 | 6.5 | 16 | 7.4 | Remains at 0% | 10.3 |
| First relapse post RTX (months) | 12 | 10 | 20 | 16 | Relapse-free | 15 | Relapse-free | 43 |
| Time to steroid discontinuation after start of RTX (meses) | 1 | 0.9 | 0.7 | 2 | 2.4 | 0.6 | 0.2 | 3 |
| Current condition of patient | ||||||||
| Follow-up (months) | 40 | 12 | 53 | 69 | 56 | 18 | 5 | 97 |
| Complications | No | No | No | No | No | No | No | No |
| IS dose in last 3 months | 0 | 0 | Steroids: 2.02 | Steroids: 2.96 | 0 | Steroids: 2.60 | 0 | Steroids: 0.56 |
| % reduction in IS | 100 | 100 | Steroids: 95.3 | Steroids: 91.5 | 100 | Steroids: 85.9 | 100 | Steroids: 98.9 |
| CPA: 100 | FK: 100 | CPA: 100 | MPM: 100 | |||||
| Steroids in last visit* | 0 | 0 | 2.02 | 44.40 (relapse) | 0 | 11.70 | 0 | 0.56 |
CPA, cyclosporine A; FK, tacrolimus; IS, immunosuppressant drugs; MPM, mycophenolate mofetil; RTX, rituximab.
Complete B cell depletion (CD19) after the initial dose of RTX was confirmed in all patients but 1 in whom the count was 1%. The median duration of B cell depletion was 10.3 months range, 6.5–16 months), and only 1 patient experienced relapse of NS during the B cell depletion period.
Following administration of RTX, we found a reduction in the frequency of relapses (3.6 relapses/year in the year before treatment initiation vs 0.1 relapses/year in the first year post RTX). In patients that experienced relapses after initiation of RTX (75% of the total), the 1-year relapse-free survival post RTX was 83.3%. The median time elapsed to the first relapse after the first dose of RTX was 15.5 months (range, 10–43). Thus, the median time elapsed from evidence of B cell reconstitution and the first post-RTX relapse was 7.8 months (range, 1–32.7).
Treatment with immunosuppressive drugs was discontinued in every patient at initiation of treatment with RTX. In half the patients, especially the first to receive RTX, mycophenolate mofetil (400−600 mg/m2/12 h) was added from 3 months after the first infusion of RTX and was maintained without interruption until the next relapse occurred. This approach was based on previous studies4–6 that found improved efficacy with the combination of RTX and mycophenolate mofetil, as the latter delayed B cell recovery, an outcome that was corroborated by our findings (P = .05). Initiation of other immunosuppressants, other than steroids, was not necessary past this point (or, in the remaining 50% of patients that did not receive mycophenolate mofetil, from the beginning). When we compared the dose of steroids that patients received in the 3 months that preceded initiation of RTX to the dose received in the last 3 months of follow-up (median duration, 46.5 months; range, 5–97), we found a 96.5% reduction (mean of 28.5 mg/m2/day vs 1 mg/m2/day).
Adverse eventsThree patients (37.5%) experienced mild adverse events during infusion of RTX (1 bronchospasm, 2 cutaneous reactions). In all 3, the side effects resolved after temporary discontinuation of the drug, and the infusion of RTX could be completed later on. We did not find documentation of infectious complications or any other complications attributable to treatment with RTX.
DiscussionEvidence from recent studies show that treatment with RTX is effective in children with difficult-to-treat N, both in terms of the frequency of relapses and its steroid- and immunosuppressant-sparing effect. Although the methodological limitations of our study should be taken into account (retrospective design), our findings in patients treated with RTX seem to confirm it.
On one hand, we found a marked reduction in the frequency of relapses after treatment with RTX, which declined from 3.6 relapses/year in the year preceding the first cycle of RTX to 0.1 relapses/year in the first year post RTX (during which 7 of the 8 patients in the study were free of relapses). These benefits of RTX in the management of NS have been described repeatedly in recent years,7–16 and some authors have even proposed using RTX as a first-line drug for treatment of steroid-dependent NS,17–19 based on the comparison of its efficacy with the efficacy of other immunosuppressant agents used habitually for management of this disease. A multicentre, double-blind, randomized and placebo-controlled trial is currently underway to assess the efficacy and safety of RTX for treatment of childhood-onset, early-stage uncomplicated frequently-relapsing or steroid-dependent NS in patients that have not previously received other immunosuppressant drugs.20
On the other hand, treatment with RTX allowed a reduction in the use of other immunosuppressant drugs; only steroids were used after initiation of RTX, and there was a 96.5% reduction in the steroid dose in the last 3 months of follow-up. These outcomes were similar to those reported by other authors8 and are particularly relevant if we take into account their use in patients with difficult-to-treat NS, who in most cases are refractory to multiple first- and second-line treatments.
Since the use for treatment of NS is not included in the summary of product characteristics of RTX, the exact indications and optimal dosage for this purpose have yet to be established, and the dose per cycle, interval between cycles and total number of cycles vary widely between published sources. Our patients were treated with a standard dose of RTX of 375 mg/m2 (body surface area) and, with the exception of a patient that received 4 weekly doses in one cycle, received 1–2 weekly doses per cycle in the schedule used most frequently by other authors.8,20 This regimen could be a valid and effective option to use from the beginning, as opposed to daily doses of other immunosuppressants, in cases in which there are concerns about adherence.
Several studies4,21–23 have found a direct association between the number of doses of RTX, the duration of B cell depletion and the decrease in the risk of relapse. This trend, which was also found in our patients, has led to specific recommendations regarding the immediate administration of additional doses of RTX once there is evidence of B cell recovery without awaiting a relapse,24 although this is not currently widespread practice. At the same time, another clinical phenomenon that has been described in relation to treatment of NS with RTX is a tendency toward earlier B cell recovery associated with younger age,4 which our findings seem to corroborate. Last of all, our findings also corroborate the positive corelation between the age at initiation of RTX and the duration of B cell depletion (and therefore of relapse-free periods) described in previous studies.4,11
Still, the association between B cell recovery and the recurrence of disease in our patients was not categorical, as there was a significant gap between both events (more than seven months). This suggests that RTX has mechanisms of action that are not directly dependent on the B cell count, something that has been described previously.25–27 In our series, additional doses of RTX were not administered in isolation based solely on the biological marker of B cell recovery, and the occurrence of a new relapse, following evidence of B cell recovery, was the clinical situation that prompted prescription of an additional cycle of RTX. Other biological markers (such as changes in T cell subsets) may be useful in the management of patients with NS managed with RTX.27
Several studies have found an increase in the effectiveness of RTX when combined with mycophenolate mofetil,4–6 something that is also suggested by our results, despite the small number of patients. Recently, Takahashi et al.7 found similar results with the use of mizobirine (a purine inhibitor) pulse therapy following treatment with RTX. At present, this is not widespread practice in nephrology, and its indication has yet to be properly established.
By the end of the post-RTX follow-up period (median, 46.5 months; range, 5–97), there had been no evidence of significant adverse events attributable to RTX in our patients, infectious or otherwise. Needless to say, the relevance of this outcome and the inferences we can make about potential long-term implications are limited by the small number of patients included in the study and the duration of follow-up.8,11,28,29
In conclusion, RTX achieved favourable outcomes in our series of paediatric patients with steroid-dependent NS, corroborating its efficacy and supporting its consideration as an appropriate pharmacological agent for use in difficult-to-treat cases. Although we did not observe adverse events or manifest infectious complications associated with treatment with RTX in the short term, adequate studies are nevertheless required to establish the optimal regimen (dosage, interval between and total number of cycles), the clinical impact and the potential adverse effects of RTX in the long term.
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Guzmán Morais B, Ordóñez Álvarez FÁ, Santos Rodríguez F, Martín Ramos S, Fernández Novo G. Tratamiento con rituximab en pacientes pediátricos con síndrome nefrótico córtico-dependiente. Experiencia en un hospital terciario. An Pediatr (Barc). 2022;96:83–90.