Introduction
The use of oxygen was implemented in neonatal practice in the absence of any randomized studies. The dose was (and still is in some places) not measured well. Additionally, the newborn's oxygenation levels were not measured routinely until Arterial Blood Gases (ABG's), capillary samples, transcutaneous PO2 (TcPO2) and, more recently, monitoring of oxygen saturation by pulse oximetry (SpO2) became available. However, it is one of the drugs most frequently used in NICU's, many times without any limits or control, as repeatedly mentioned in books, editorials and articles, as recent as December 2003 and February 20041,2. The relationship between PaO2 and saturation is a "changing" one, as reported many years ago. The relation of prematurity, oxygen and ROP has been studied by many3-8. However, over the past 63 years, the history of this condition and these relationships continue to evolve. It is our objective to bring up to date some of the important topics in these evolving and changing relationships. In this review we will include, in greater or lesser detail, the definition ROP, the grades of ROP severity and the unsolved issues of its treatment, a few historical aspects, and the population at risk and associated risk factors. We will also discuss the difficulties with accurate statistics in ROP (rates and intercenter and inter-nation variability), the significance of the problem, and the pathophysiology in the developing retina. We will end by providing the best available evidence of the relationship between different oxygenation levels (PaO2 and TcpO2; oxygen saturation, SpO2) and of rapid changes and "fluctuation" of oxygenation on ROP. We will also describe how a process of education and of implementation of guidelines to accomplish changes in clinical practice is associated with a clinically significant impact in the prevention of some of the most severe cases of ROP. To end, final comments and a few future studies currently in planning stages will be briefly discussed.
What is rop?
Definition and Grades of Severity
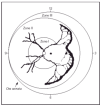
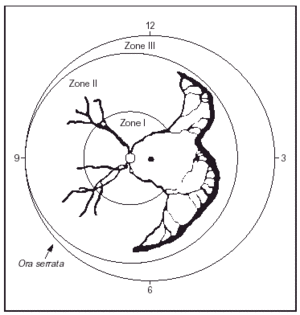
ROP is a developmental abnormality of the retina and vitreous that occurs in premature infants. It is due to abnormal angiogenesis, in which retinal blood vessels fail to grow and develop normally in infants born prematurely, sometimes resulting in visual impairment and blindness. The disease is associated with, and affected by, survival rates and severity of systemic disease. It is of variable severity, classified after detailed ophthalmologic fundoscopic examination with INDIRECT ophthalmoscopy. An International Classification (ICROP) has existed for many years9. The classic stages described by ICROP are of increasing severity (stages 1-4). The eye is divided in zones, according to which area is affected. Zone I ROP occurs when at least one clock hour (sector) of ROP is seen within an imaginary circle in which the radius is twice the diameter from the optic disk to the macula; is the most central zone, close to the optic nerve, and most critical to central vision. It is for this reason worse zone to have abnormal vessel formation. Zone II ROP -intermediate zone, occurs when retinal vessel maturation had not occurred to within 1 disc diameter of the nasal ora serrata. Zone III ROP (peripheral) occurs when retinal vessel maturation is to within 1 disc diameter of the ora serrata on the nasal side, in at least 2 clock hours. Figure 1 is a schematic representation, where "clock hours' of area affected are also represented. Furthermore, terms like threshold; prethreshold and "plus" disease are used. Threshold is used when treatment is indicated. Prethreshold [laces the infant at very high risk for treatment. Plus disease requires at least two quadrants of dilation and tortuosity of posterior pole retinal blood vessels equal to or exceeding that of a standard published photograph. In addition, the ETROP study, published in December 200310, put forth the concept that flat neovascularization in Zone I, even without an actual ridge, should be considered Stage 3 and proposed two Types (I and II) to decide on the more severe cases and therapy10, based on zone and on the presence or absence of plus disease as follows:
Figure 1. Cartoon of ROP in Zone II with extension of more than 5 contiguous hours. (From Reynolds JD, et al: N Engl J Med 338:1572, 1998.)
1.Type 1 (most severe):
a) Zone I, any stage ROP with plus disease
b)Zone I, stage 3 ROP with or without plus disease
c)Zone II, stage 2 or 3 ROP with plus disease
2. Type 2:
a) Zone I, stage 1 or 2 ROP without plus disease
b)Zone II, stage 3 ROP without plus disease
Treatment and its effectiveness
Unfortunately, to date there is no completely effective "cure" for ROP nor a treatment to stop its progression to the most severe forms. Cryotherapy was used in the past for treatment but laser therapy is the current preferred treatment.
The largest study with published to date with a natural history cohort reporting incidence of ROP is the CRYO-ROP study11, however more modern data regarding incidence will soon be reported from the ETROP efforts. The infants in the CRYO-ROP study were born in the USA between January 1986 and November 1987 and the reported incidence in infants < 1,251 grams was 66 %. The results showed that cryotherapy was beneficial but many children were left with significant vision loss even after this procedure.
The ETROP study10 screened 6,998 infants < 1,251 gm in 26 centers in the USA. The infants with high risk pre-threshold ROP where randomized for early versus conventional treatment. The results showed that earlier laser treatment was associated with a reduction of unfavorable visual results at 9 months post conceptional age (PCA): 19.5 % vs 14.5 %. However, there wa no statistical difference in relation to blindness. And there were more 'complications' during the earlier procedure. By analyzing the data, one can estimate how many would be treated unnecessarily: Type I in cohort: 37 % with normal structure never reached threshold. This means that if all infants with type I are treated, for 3 that are treated 1 would not have required the procedure.
Two recent controlled randomized and blind trials assesses whether a strategy of "additional oxygen" in infants > 1 month old reduces progression of retinal damage once the disease process is well underway. The Supplemental Therapeutic Oxygen for Prethreshold ROP trial (STOP ROP)12. included infants of about 26 weeks at birth who developed prethreshold ROP in at least one eye and who could not maintain SpO2 > 94 % on room air. Infants were randomized late in the neonatal course once the diagnosis of threshold ROP had been made to one of two groups: supplemental O2 to maintain higher SpO2 target (SpO2 96-99 %) versus 'conventional' SpO2 (SpO2 89-94 %). The study failed to show a significant benefit for supplemental oxygen. Progression of prethreshold ROP to threshold was reduced by 28 %, but the difference did not reach significance (OR 0.72, 95 % CI 0.58, 1.01). A post hoc subgroup analysis of prethreshold ROP without plus disease revealed lower risk of progression to threshold among the tiniest infants in supplemental group (46 % to 32 %, P = 0.004)12. However, the reported side effects were much worse in the higher oxygen saturation group. These included pneumonia, exacerbation of BPD, adverse pulmonary events through 3 months corrected age, prolonged need for supplemental oxygen at 50 weeks PMA, and significantly increased use of furosemide. The Australian trial Benefits of Oxygen Saturation Targeting (BOOST)13 randomized 358 infants < 30 weeks gestation who were O2 dependent at 32 weeks post menstrual age. The oxygen saturation target was 91-94 % in the "Standard" group and 95-98 % in the High Saturation group. There was a consistent trend but no statistical difference toward reduced need for retinal ablation in higher vs. lower SpO2 group (RR 0.5, 95 % CI 0.3-1.1, p = 0.09). In infants born < 28 weeks gestation (n = 256), ablation was reduced by 48 % in the higher SpO2 group (RR 0.52, 95 % CI 0.26, 1.03). There was no evidence that any of several outcomes (hospital stay; growth; major developmental abnormality; neurodevelopmental at 12 months corrected age; parental stress and infants temperament) were changed. Duration of O2 therapy & IMV were not improved either. Actually, BPD (O2 at 36 weeks) was higher in those in the high saturation group (64 % vs 46 %) and a greater proportion was discharged home on supplemental oxygen (30 % v 17 %). There were also more respiratory deaths in the higher SpO2 group13. These two trials found no difference in growth, mortality, or major developmental outcomes.
Therefore, the STOP ROP study12 failed to find convincing evidence that giving additional oxygen significantly reduced the rate at which retinal damaged progressed once this is well underway and the BOOST13 trial suggests that higher SpO2later in the neonatal course may have some role in reducing the progression of existing ROP in some premature infants. More importantly both illustrate potential tradeoffs in safety and efficacy of specific ranges of SpO2 for different outcomes (i.e.: respiratory versus visual). With small increments in FiO2 there was evidence for increased pulmonary sequelae and no detectable benefit in growth and neurodevelopmental outcome.
In summary, there is still a need to find a cure and to identify the "right time to treat", so no missed opportunity exists while at the same time no infant is treated unnecessarily. As importantly, when the diagnosis is made, there is still no treatment that would effectively stop the progress to more severe forms. Above all, there is no definite and completely preventive measure, short of eliminating premature births. Data of STOP ROP and BOOST are important, but they are not relevant to address the optimal SpO2 level early in the neonatal course which would result in less frequent, less severe ROP and less chronic lung disease. We and others have reported that careful monitoring of O2 administration and of O2 saturation, avoiding wide fluctuations and high O2 saturations soon after birth and for the first several weeks of age, is associated with a lower incidence of severe ROP, less need of laser treatment, less blindness and less BPD.7,8,14,15 (More details on this are described later).
A few historical aspects
Oxygen was discovered more than 200 years ago16-17. Scheele in 1773 and Priestley on August 1, 1774 discovered oxygen independently from each other. Priestley was unaware of Scheele's earlier work, since it was not published until 1777. Because of his early publication on March 8, 1775 and subsequent detailed experiments on the nature of this new gas, plus his direct influence on Lavoisier (who actually named the gas "oxygene", meaning acid-former, and who then initiated the "Chemical Revolution"), Priestley is usually credited with the discovery of oxygen16. As far as it is known, Priestley was also the first person ever to inhale air with a greater than normal concentration of oxygen. It seems that in 1780 Chaussier18 gave oxygen to babies and since then it has been given to more infants in the world than any other neonatal treatment. Interestingly, Priestley never really knew he discovered oxygen. He insisted to his death that he discovered "dephlogisticated air".. He actually was entangled in an incorrect theory and kept warning others to stick to the evidence and not give in to their prejudices. Claude Bernard, 100 years later wrote," It is the things we do know that are the great hindrance to our learning the things we do not."16. In 1917 intragastric administration of oxygen was recommended, a practice that lasted into 1950 and in 1928 Flagg described a method for resuscitation of asphyxiated neonates using oxygen and carbon dioxide18.
In the US and other industrialized nations O2 therapy for neonates was introduced in the 1930's and early 1940's. Its use then became widespread throughout the world. In February of 1941 Dr Clifford a pediatrician in Boston and Dr Chandler, an ophthalmologist, saw a child with roving nystagmus, opacities of the eyes and a "fibro-vascular sheath of the lens". Within a week another child was seen by them and Dr Terry. These two infants weighed at birth 1.02 and 1.81 kg and they were the forerunners of an epidemic of blindness, with 117 cases between 1942 and 1945. In 1944 Dr Messenger (an ophthalmologist and Latin and Greek scholar) coined the term retrolental fibroplasia ("RLF"). In the early 1950's the relation between O2 therapy and RLF (name used then for this ROP condition) was discovered. By 1953: 10,000 blinded children by RLF (7,000 born in USA) had been identified. These issues and others are described with factual evidence and elegance by Dr Silverman in his book, "Retrolental Fibroplasia: A Modern Parable", available on http://www.neonatology.org/classics/parable/default.html19. This is a must for all in leadership positions in Neonatal-Perinatal Medicine and also enlightening and inspiring for any concerned pediatrician. In brief, the O2 concentration was turned down in many incubators and there was a reduction in ROP but increased morbidity and mortality. A tragedy happened in the 1950s and 1960s19-21 when a new and ultimately harmful standard of care that was based on incomplete evidence had been widely implemented. As a few examples, McDonald reported in England that when O2 was curtailed to 2-6 days, the prevalence of spastic diplegia was as high as 25 %, but there was no RLF. When O2 was maintained for 17-25 days or more, RLF was seen in over 25 %, but spastic diplegia only in about 4-5 %. Cross20 reported on the cost in lives in 1973 and Bolton and Cross reported further details in 197421. They found that after oxygen restriction was recommended by consensus the mortality rate in the first 24 hours of age per thousand live births did not continue the significant improvement and downward trend in USA, England and Wales from the 1950's to the 1960's. In addition, based on the excess number of deaths in the first day of life they estimated that "each sighted infant gained may have cost the death of 16 infants". During those days O2 dose was not measured accurately, and it was impossible to measure levels of oxygenation. Today the relationship is different, and is still evolving.
It has been known since those early days that the proportion of severe visual impairment and blindness due to RLF/ROP in children aged 0-15 years in schools for the blind is inversely related to infant mortality rate, being much higher in industrialized than in developing nations. In data collected by a standard definition and methods between 1991-1996 in developing nations, Gilbert et al22 reported that the proportion of severe visual impairment and blindness due to ROP in children aged 0-15 years in schools for the blind was 30 % in Cuba, 18 % in Chile and only 4 % in Guatemala, associated again with better infant mortality rates.
Of course much has happened in this last 25-40 years. Blenders became available, as did accurate and simple to use oxygen analyzers, giving clinicians the possibility of controlling and knowing the dose of O2 (FiO2). In the late 1960's and early 1970's PaO2 measurements became available, as did micro methods for determination of arterial blood gases. This history monitoring of O2 and blood gas is also very interesting and we have recently summarized it23. Non invasive continuous transcutaneous measurement of PO2 (TcPO2) entered practice in the mid-late 1970's and early 1980's and, as useful as it was, is phased out of use. The measurement of oxygen saturation by SpO2 monitors became available in the 1980's, but its history is still evolving.
In the past 3-4 years several editorial comments have been written on the topics1,2,24-27 and several original articles have been published just in the last 18 months7,14.
However, truly effective care based on systematic reviews of the evidence obtained from randomized controlled trials is still not possible in relation to prevention of ROP, clearly not for O2 therapy, PaO2 and SpO2 levels.
Population at risk and associated risk factors
The four major factors clearly associated with ROP are prematurity, oxygen use, male sex and white race. Of course, the problem tends to be more frequent and more severe in infants born at extremely low gestational age (24-27 weeks of gestation) and with ELBW (< 800 grams). However, the disease still exists in many regions of the world in infants who are ≥ 32 weeks' gestation and > 1,750 grams. This was the case 30-40 years ago in industrialized nations, where now ROP is non existent in this gestational age and birth weight groups. These factors lend more support to the impact of clinical practices on the development of severe ROP.
There are many other factors that have been postulated as associated risk factors, but they have not been clearly confirmed or proven in large well performed studies. Discussing each of them in any detail escapes the objective of this review and they will only be named and only some briefly discussed below. The multiple factors explored in association with ROP include hypoxemia, indomethacin therapy, vitamin E and A deficiency, inositol, patent ductus arteriosus, lack of breastfeeding, postnatal steroids, light exposure, candida sepsis, numerous blood transfusions, early use of iron, the use of erythropoietin (EPO) and hypercapnia.
Post natal dexamethasone may be associated with severe ROP28-31. In a randomized trial of ELBW infants (750 grams) ventilator dependent at 15-25 days of age it was associated with a higher proportion of severe ROP (56 % vs 45 %) and need for ablation (35 % vs 20 %)30. Light reduction was not associated with ROP prevention32. Several clinical reports associate candida sepsis with a significantly higher proportion of severe ROP and surgical therapy for ROP31,33-35. The effect of blood transfusion on retinopathy of prematurity is still controversial36-38. A prospective, randomized study, combining data from both groups, showed no association between hemoglobin hematocrit ratios or transfusion protocol and ROP incidence or severity36. Dani et al38 has nicely described the potential role of blood transfusions and iron intake on ROP. In preliminary reports presented at research meetings it is suggested that EPO use for anemia of prematurity may be associated with an increase incidence of ROP39,40. In relation to hypercapnia and apnea and their potential role in ROP we refer the authors to the section on pathophysiology described later.
ROP "Statistics" (rates and intercenter and inter-nation variability), Eye Examination and Significance of the problem
Unfortunately in many NICU's in the world the rates of ROP are still unknown or the statistics of this condition are inaccurate. This is due to several possible reasons. First and foremost, if neonatal mortality for VLBW is high, the rates of ROP will be "low", particularly so in units that report cases with ROP in relation to admissions. Secondly, with any VLBW mortality rates, the data for ROP should not be reported per VLBW infants admitted to the unit. The numerator should be the number of infants with ROP and the denominator should only include screened infants. A summary of how data should be reported to allow valid comparisons is shown in table 1. Finally, for accurate statistics on this condition, all babies at risk must be examined and screened correctly before discharge and the screening rate known in the NICU (table 1). If this is not done, the true rates of ROP in a particular unit will not be accurately known, and could be falsely low and comparisons among different units will not be valid.
The issues mentioned above are related to the variability that exists between centers and of course between regions or nations. However, when all steps are followed, there is still significant inter-center variability. In several large data bases one observes that the rates of severe ROP (III-IV) vary from 2 % to more than 12 %, and the need for laser treatment varies between 1-4 %. This inter-center variability is related to differences in clinical care of oxygen administration and monitoring, and other aspects of care. These differences in care also explain, at least in part, within center variability from one epoch to the next.
To ensure accurate data, the infants considered at risk should be screened following a detailed protocol so that (almost) no infant is missed. Additionally, the eye examination should be methodically performed. The infant's pupils must be dilated (cyclopentolate 0.2 % and phenylephrine 1.0 %; cyclomydril) to ensure thorough examination. If there is poor dilation, cyclopentolate 0.5 % and or phenylephrine 2.5 % can be used. The exam involves the use of a sterile eyelid specula and scleral depressors in order to visualize the peripheral retina with a binocular indirect ophthalmoscope, along with a hand-held lens. The infants must be carefully monitored for signs of distress caused by the examination.
There are also guidelines as to when to repeat follow up exams. If there is no ROP during first exam, the infants need to be examined until retinal vessels had developed. In eyes in which ROP is observed, the location and severity must be recorded according to ICROP9. Zone I ROP needs to be examined every week. Diagnosis of Zone III ROP requires follow up at least once, 2 weeks later. Zone II needs to be followed every 1-2 weeks until full maturity or worsening is found.
The significance and impact of ROP is crucial. About 4-5 % of survivors < 1,000 grams are legally blind. A larger percentage has significant visual impairment. If one accepts a gross estimate that each year there are 2,000 VLBW infants discharged alive from NICU's in the USA who are blind or severely visually impaired and that the life expectancy for those infants is 70 years, one can calculate that each year there are 140,000 new years of blinded life entering society. Severe ROP not only leads to blindness but is associated with severe abnormal neurodevelopmental abnormalities. VLBW infants with severe ROP fare much worse than those without visual impairment. As ROP severity increases the severe disability rate increases from about 4 % to 20 % when there is threshold ROP. More than half of the infants with unfavorable vision have severe disability. With unfavorable vision the functional outcomes are worse, with 77 % being unable to provide self care, 50 % having problems of continence, 43 % motor disabilities and 66 % altered personal-social skills, 3-10 times more frequent than controls with favorable vision41.
Finally, in almost every region that data is obtained from schools for blinded children or adults, the proportion of severe visual impairment and blindness due to RLF/ROP is much higher any other cause (or many causes combined). ROP in these settings is the most common cause of blindness. The significance of this problem is also now being reported from developing nations, where survival of VLBW infants is increasing and a large numbers of infants with severe ROP and/or blindness are entering society42,43). The saddest part is that many of these infants are "large" (i.e. > 27 weeks gestation, or > 1,250 gm at birth), similar to what happened, but no longer occurs, in industrialized nations 30-55 years ago.
Pathophysiology and the Developing Retina
The two triggering factors are an incompletely vascularized retina (preterm infants) and increased PaO2 with relative retinal hyperoxia. These leads to vasoconstriction and decrease in growth factors, among them are insulin like growth factor (IGF-1) and vascular-endothelial growth factor (VEGF). This leads to arrest of vascularization and capillary obliteration, which leads to decreased perfusion and subsequent retinal ischemia and hypoxia. In response to this, various growth and angiogenic factors (IGF-1, VGEF and others) are upregulated. If this response is marked and vasogenic factors do not decrease, angiogenesis (i.e.: new blood vessel formation) becomes abnormal and disorganized with significant vasoproliferation. This could finally lead to inflammation, proliferative retinopathy, significant fibrosis and retinal detachment44-50.
Even though hyperoxia and the formation of radical oxygen species are clearly a predominant part of the pathogenesis, other factors like pro-inflammatory cytokines, cyclooxygenase-2 (COX-2), neuropeptide Y, Nitric Oxide (NO) and deficit of trophic factors and anti oxidants have been implicated. COX-2 has pro-angiogenic effects mediated by prostaglandins (PGE2) and activation of specific receptors (EP3 receptors). This leads to induction of eNOS expression, the endothelial form of the NO synthase, which would lead to more NO being present. This is why some investigators are exploring COX-2 inhibition to attenuate intra vitreal neovascularization. The neuropeptide Y has 36 amino acids and is upregulated by hyperoxic exposure. Depending on the time a, significant neovascularization can occur46-48. Interestingly, in a genetic "knockout" model of Y2 receptors, there is significantly less retinopathy, blood vessel tuft formation, retinal hemorrhage and blood vessel tortuosity despite breathing FiO2 75 % for five days after day 7 of age48. Cyclooxygenase-2 (COX-2) is involved in neurodegenerative events in the rat retina49. The role of the soluble receptor of tumor necrosis (sTNFR) was recently evaluated in 14 VLBW infants in a pilot study. It was found that infants who later developed severe ROP had higher serum levels of sTNFR between 3 days and 5 weeks of age50. These findings are leading investigators to study different forms of inhibition in an attempt to attenuate abnormal neovascularization.
Regarding hypercarbia, Holmes reports on carbon dioxide-induced retinopathy in the neonatal rat51. Furthermore, recent reports in animal model and tissue cultures show that hypercarbia without any hemodynamic effects increases NO synthase (NOS) isoforms in the retinal vasculature, resulting in cytotoxicity of the vasculature52,53. When hypercarbia coexists with elevated NO in the retina, the retinal lesions are many fold worse. In addition it has been clearly demonstrated that hypercarbia induces vascular development and prolongs activation of endothelial NOS52,53. Finally, the role of VEGF and IGF-1 in a hypercarbic oxygen-induced retinopathy rat model of ROP has been recently described54. The clinical information is incomplete and non conclusive55,56, but this is not unexpected due to the multifactorial issues involved in the pathogenesis of ROP.
As clinicians we can observe our practices and evaluate if any changes introduced in clinical practice before there is 'enough evidence' of safety and efficacy have any impact on important outcomes. Nowadays many NICU's allow some very ill infants to remain on CPAP early in the infant's life, even if they have high PaCO2 and show significant oxygen fluctuation 'down and up' due to intermittent apneic episodes of variable severity. In those cases we are able to document only intermittently a few, limited variables like PaO2 and PaCO2, but as clinicians we cannot see what is happening, if anything, with NOS, NO, COX-2, IGF and VEGF systemically or in the retina. Interestingly, in addition to GA and BW, it was recently reported that apnea and surfactant therapy are significant independent risk factors for ROP. Furthermore, apnea may not only increase the risk of developing ROP, but may also worsen pre-existing ROP57.
Until further evidence is made available, the rates of ROP need to be monitored carefully and accurately if clinical practices are modified. The question today is which among the factors related to hypercarbia, hyperoxia and significant and rapid oxygen fluctuation has a greater or lesser impact on ROP. They may all modify the expression of some or all of the factors mentioned above, and may be more detrimental in combination when the infant is more immature and the retina significantly underdeveloped. Unfortunately, it seems that there are regions and centers in the industrialized world which are showing an increase in severe ROP58-60, NOT associated with an increased survival of the tiniest infants. One can only wonder and speculate if the introduction of several new practices has a relation with this. Surfactant came in early 1990's and can be associated with rapid improvements in oxygenation. Around the same time, SpO2 monitors were introduced. In many centers high SpO2 values were routinely allowed and, therefore, a lack of a fast enough response to the surfactant induced changes in oxygenation could be associated with very high (unknown) PaO2 levels for varying periods of time early in post natal life. This, in addition to the rapidly fluctuating changes in oxygenation associated with the use of SpO2 monitors may explain in part the increase and variability in rates described before. CPAP with significant hypercarbia and frequent episodes of low and high oxygen levels early in life may also have an impact on ROP.
Oxygen Administration, Oxygenation Levels, Rapid Changes and ROP
Oxygen was discovered more than 200 years ago (see before) and it has been administered to more infants in the world than any other neonatal treatment. However, we still do not fully know how much is wise to give or how much infants actually need in relation to variations in illness and gestational and postnatal age. But we have known for many years that "too much oxygen" damages the retina3-6,61-64.
Why choosing between the 'extremes" of oxygen dose: 21 % (room air) versus 100 % ('pure oxygen')?
In many places, and even in the recent literature, there is a longstanding debate about using 21 % oxygen (FiO2 0.21) versus 100 % or 'pure' oxygen (FiO2 1.0) during resuscitation65-72. This practice of choosing 'one or the other extreme' cannot be a correct practice for many infants since it excludes or limits a more reasonable practice, a practice based on using an 'adequate' dose of oxygen, assessing each infant's needs. Placing a pulse oximeter, targeting for an "acceptable" SpO2 and using a blender to be able to administer the needed oxygen dose for that target are simple and inexpensive care measures, which are used 'day in and day out' in every NICU in many nations. Measuring FiO2 and O2 saturation (and/or) ABG's is done routinely in many NICU's. Why is this not done routinely from the time of birth in preterm infants treated in many places through out the world? Why is this practice not done consistently during resuscitation, and at any time in the delivery room, in hospital transit or during hand ventilation? Not to do so is not supported by any literature nor by physiological evidence.
It is well known that resuscitation without oxygen is effective as described by the references just cited above. Additionally, mouth to mouth resuscitation delivers less than room air (FiO2 19-20 %) and has been known to be effective for decades. The neonatal practice of 'one or the other extreme' (21 % vs 100 %) in the delivery room may be understandable in poor, underdeveloped areas, with no expensive technology and no NICU's. In such places (and only in such places), if severe lung disease is unlikely or ruled out, it seems that the best alternative would be to resuscitate with room air, and change to 100 % FiO2 in the particular infant who shows no good response. Or alternatively, if in doubt, it may be necessary to start with 100 % and wean to room air immediately after signs of initial recovery start to be evident. In any other place in the industrialized world and also in centers in developing countries that are able to afford and have modern expensive technologies and NICU's, practicing with only two extremes of O2 dosing (21 % versus 100 % O2 administration) is just suboptimal.
Oxygenation levels and 'bad practices'
Currently oxygenation is measured in neonates in one of two ways: arterial blood gases (PaO2) and pulse oximetry (SpO2) monitoring. The history of oxygen monitoring is also very interesting and has been recently summarized23. Transcutaneous PO2 electrodes were used in the 1970's and, as useful as they were in the past, they were replaced by SpO2 in the mid to late 1980's. Capillary O2 (PcO2) determinations are not reliable to measure oxygenation, since a PcO2 of 45 mmHg may represent a PaO2 of 50mmHg or one > 80-100 mmHg. SpO2 monitors are currently widely used and this history is still evolving as new technological advances are entered into practice. In a separate review on pulse oximetry in neonatal medicine73, we describe the most important aspects of SpO2 and the significant differences in saturation monitors in the market, and how much we know and do not know about oxygenation. Suffice to say here that as with any measuring device there is an accepted variability of ± 0.5 % to up to 3 % when compared to the 'gold standard' (Co-Oximetry). Additionally, one SpO2 monitor is not the same as another SpO2 monitor. Many SpO2 monitors have high rates of false alarms, are not accurate in eliminating noise, have 'holding periods' and are more sensitive to light and motion artifacts. In summary, many SpO2 monitors may not function well when clinicians need them the most. In addition, some SpO2 monitors measure and display functional O2 saturation and some fractional O2 saturation73,74. Some monitors read 1.5 %-4 % higher or lower than other monitors, even in very stable conditions. Therefore, this information helps us understand that finding the 'ideal' or best O2 saturation in preterm infants is not easy, and cannot be just one value.
In many places the oxygen dose (FiO2) is not measured carefully all of the time when given to premature infants, due to lack of blenders. The lack of use of blenders in the delivery room, providing 100 % (FiO2 of 1.0) and no measurement of O2 saturation is unacceptableas we have described in a Pediatric textbook75. In such cases, unless the lungs are severely affected, the PaO2 could be very high (i.e.: 200-400 mmHg) and the real arterial O2 saturation would be 100 % with an SpO2 reading of > 95 % (up to 100 %), if measured. Until true evidence is available, care providers can argue and struggle about what is the best, ideal, SpO2 level and disagree if the "target saturation" for weaning the FiO2 should be 93 % or 96 % or some other %. But this can be done with humility, cognizant of our ignorance of what is "best" and of the caveats regarding monitor's measurement errors and differences between SpO2 monitors73. But what ever these and other limitations of our current knowledge may be, using a blender and a measurement of oxygenation (i.e. SpO2 monitor) 'increases the evidence'. In such way, the infant would not be exposed to definitely abnormally high oxygenation levels and the FiO2 would be weaned as rapidly as necessary, as tolerated by the infant.
Another 'bad practice' is that of manual ventilation in an intubated infant when this is done with gas flowing into a breathing bag directly from the wall O2 flow meter (i.e. 100 % or FiO2 1.0). For many reasons, like intubation in the delivery room, "deterioration" in NICU, change of ET tube, and others, manual ventilation may be necessary. However, we must remind ourselves that the gas "from the wall" is 'pure oxygen' and is cold and dry. These last two issues will change flow dynamics and affect the lung and the airways. In addition, if the infant was previously receiving FiO2 < 1 (i.e. 40 %; 60 %; 70 %) and was stable, and now, with this practice of manual ventilation with 'pure oxygen', without a heater humidifier and a blender, the saturation reads 100 %, it is impossible to know how high is the infant's PaO2.
Yet another bad practice occurs in many places when the dose of oxygen (FiO2) is changed significantly without written orders or documentation. Even today, in many places and during many NICU shifts, the FiO2 is modified significantly during some periods of time by care providers caring for preterm infants. For the most part, these modifications go undocumented in the clinical records. This delivery of clinical care with risk for "abnormally high" FiO2 and oxygenation levels is described in the following circumstances. A preterm infant in NICU shows a decrease in O2 saturation (i.e. SpO2 < 75 %-80 %) and the monitor alarms. The care provider increases the FiO2 by 5-10-20-30 % or more. The infant then "recovers", the SpO2 reads 98-100 % and the infant is left in "good condition", since the O2 saturation is "as good or better" than before. Many infants are left like this, without accurate documentation. And many monitors' alarms are turned off. Most infants, if not all, do not have an ABG measured during the period just described, when the FiO2 has been changed significantly. Subsequently, minutes or hours later, when the infant is found by another care provider with an increased FiO2 and an SpO2 of 97-100 %, a frequent practice is that the FiO2 is immediately brought back to, or close to, baseline levels. The infant may then be "well oxygenated" with O2 saturation of 93 % and PaO2 of 45-60 mmHg or "rebound" to low SpO2. Therefore, in infants breathing FiO2 > 0.21 who are exposed to these (too frequent) circumstances, we do not know how high the PaO2 was during those times when the PaO2 was not measured but the O2 saturation was 97-100 %. Additionally, the infant may be subject to wide fluctuations in oxygenation. This is what we have come to call 'A LOST RELATION'. A similar situation is observed with the use of nasal cannulas. An infant is with 0.5L/min and FiO2 0.30 or so, the infant "desaturates" and or has an apneic episode and the SpO2 monitor alarms. The FiO2 is increased to 0.40 or more and/or the flow rate is changed. The saturation "recovers" and reads higher. The infant is left "in good condition" with such treatment. How high is the PaO2 level?
If during fetal life growth and health occur with saturation of 70-80 %, why have we insisted to maintain oxygen saturation > 95 % in preterm infants receiving supplemental oxygen? This is of particular significance since the pulse oximeters do not give enough information about the blood oxygen tension (PaO2), which could be very high (i.e. > 200-350 mmHg) when an infant breathes supplemental O2. Oxygen saturation monitors were entered into practice in the 1980s not only without randomized trials but also, and probably more importantly, without education of bedside care providers around the world (i.e.: nursing staff, respiratory therapists and also physicians) about the changing relation between O2 and Hemoglobin, PaO2 and O2 saturation. When a preterm infant (and any human being) breaths a gas with oxygen supplementation and has an O2 saturation of 100 % the oxygenation levels and PaO2 cannot and be predicted, and it can be "as low as" 60-70 mmHg or "as high" as 400 mmHg, or higher. Despite all this, in many places "physiological" targets of O2 saturation were accepted and entered into practice for preterm infants of all gestational ages and postnatal ages receiving supplemental oxygen based on O2 saturation levels seen in healthy term or preterm infants breathing room air (median values 96-99 % as described in references 76-79).
The optimal or ideal SpO2 for preterm infants is not known. Therefore evidence based clinical practice is impossible. However, since so many babies receive O2 and are monitored with SpO2 monitors, we should make an effort to identify and try to eradicate 'bad practices' like the ones described here.
What is the evidence that high PaO2 and SpO2 levels and widely fluctuating levels are 'bad practices?
A cohort study in Northern England in relation to infants born between 1990-19947 found that infants treated with the intention to maintain the SpO2 target between 80-90 % had similar rates of survival (around 50 %) and CP (15-17 %) at 1 year of age than those infants maintained at 88-98 %. However, only 6 % in the first group had threshold ROP versus 28 % in infants with SpO2 in the upper ranges. Additionally, the infants with lower SpO2 limits had less days of O2 (40 vs 96 days) and IMV (14 vs 31 days), and their weight fell much less, with only 17 % weighing less than the third percentile at discharge, compared to 45 % in the high SpO2 ranges. There were 295 surviving infants, but the policy regarding SpO2 was found out retrospectively and 4 different types of monitors with different techniques were used.
Several surveys reveal similar situations. The most recent one14 reported that the range of SpO2 for VLBW infants varied from 82 % to100 % among all responders. The mean minimal SpO2 was 90 % (± 3); the mean maximal SpO2 96 % (± 2) %. The proportion of NB with SEVERE ROP (≥ 3) was lower if the objective of maximal SpO2 is < 92 % (2.2 % VS 6 %). Retinal surgery in VLBW infants is less frequent when SpO2 ranges are < 98 % in the first two weeks of age (2.5 % vs 5 %) and also less frequent if SpO2 is < 92 % even after 2 weeks of age.
Bornhorst and Poets80 have shown that using an upper alarm limit of 95 %, there is 95 % sensitivity with a Masimo® oximeter for detecting a PaO2 above 80 mmHg, currently the upper limit recommended by the American Academy of Pediatrics81.
Marlow, commenting on future clinical trials mentioned that SpO2 climbing too high should be avoided and probably not accepted by ethical committees82. After intensive and extensive work of Dr Cole, to try to initiate the POST ROP study, the saturation ranges proposed for the two groups (85-89 % and 91-95 %) do not include a group of "high O2 saturation" (i.e. > 95 %). Finally, we have recently showed that a strict policy of oxygen administration and monitoring, targeting O2 saturation 88-93 % is associated with a significant reduction of ROP8, described in more detail below. Avoiding high oxygen saturation and the possibility of hyperoxia (high P O2) is clearly supported in all current studies. Even though we still do not know how low of a SpO2 is safe, we do know that saturations of 88-93-95 % will maintain PaO2 above 45 mmHg and usually less than 75-80 mmHg. Whether this PaO2 is too high for some very immature babies remains to be determined, but this concern is what lead some clinicians to try maintain SpO2 < 92 % in the most ELGA infants.
Avoiding hyperoxia may be beneficial for conditions other than ROP, such as BPD and pereiventricular leukomalacia. Additionally, oxidative stress influences apoptosis and cell growth83. This may have some relation to white matter injury and to long term consequences in growth and development and even in carcinogenesis. In this regard it has been reported that oxygen at birth increased the risk of childhood lymphatic leukemia (OR 2.3; 95 % CI 1.5-3.6) in a Swedish study84.
In relation to fluctuating oxygen levels and ROP, several animal and humans studies lend support to the relation between fluctuation and retinal damage85-91. Penn85-87 showed that range of PaO2 and alternating values make retinopathy worse in a model of ROP. A 12 hour cycling of FiO2 (FiO2 changing from 40 to 80 %) produced neovascularization in 72 % of the cases, but while FiO2 was constant at 80 % neovascularization occurred in 18 %. The severity was of 5 clock hours in the first group of animals vs 2.3 in the latter. Saito88 found that the larger the coefficient of variation of PaO2 the worse was the retinopathy; Cunningham studied the variability of TcPO2 (31 vs 38 infants) and found worse retinopathy when the variability was larger89. Finally, York analyzed arterial blood gases in 231 infants between 1993 and 1995. On average the blood gases were measured every 2.4 h and the coefficient of variation was directly related to the risk of ROP90. Very recently, McColm has shown the effects of hyperoxic fluctuation in a rat model91.
In summary, in the past few years, a number of studies have suggested that the "physiologically normal" O2 saturation of healthy infants breathing room air, long-accepted by many as targets for SpO2 in NICU, may be too high for the premature infant, and that allowing significant fluctuation is detrimental. Unfortunately, aiming for "high" O2 saturation (i.e. > 95 %) in preterm infants treated with oxygen has been, and still is in some places at some times, a routine clinical practice, accompanied by a frequent "up and down" in FiO2. A "comfort zone" exists, and within this, it is assumed that if the O2 saturation is "high" the preterm baby is doing well. This needs to change, but the values and ranges of "lower" SpO2 saturations reported in recent cohort studies cannot be the basis for routine treatment for all premature infants of all gestational and post natal ages. No range should become established practice before questions regarding short and long-term risks and benefits are resolved and until (if ever) the same SpO2 last generation monitor technology is used universally. However, this is not the same as saying "we'll continue to deliver care as always because that is the way we have done it". Eradicating some bad practices is not the same as implementing routinely unproven practices in a rigid way. Increased awareness and education of known and proven facts must be disseminated, since the gap between knowledge and practice is associated with morbidity and can sometimes be lethal.
Changing Culture in NICU's: Process of education and implementation of guidelines regarding oxygen administration and monitoring
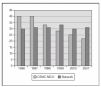
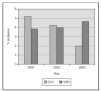
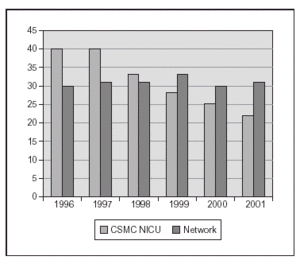
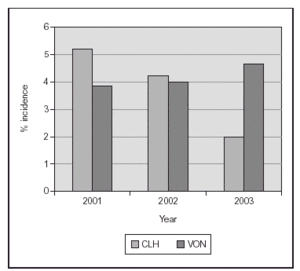
We recently published a study where a strict practice with adequate SpO2 monitoring (Masimo®) and a continuous quality improvement process of education and implementation of guidelines to accomplish changes in clinical practice were associated with a clinically significant impact in the prevention of the most severe cases of ROP8. The practice change occurred from the time of birth, with implementation of blenders and SpO2 monitoring in the delivery room, and the acceptance of "lower than usual" SpO2 values until the retina became mature. We aimed for SpO2 (85 %) 88-93 % (95 %) and to avoid wide swings in FiO2 and SpO2. In 238 VLBW infants who survived and had detailed eye examinations, the rates of severe ROP decreased from 12 to 2 % and there was no laser surgery required. This included 148 survivors and examined infants who had a birth weight of 500 to 1,250 grams8. In addition, the rate of BPD decreased significantly also (figure 2). We just had a very similar experience in the last 2 years at a different center (with a decrease from 13 % to 3 % in rates of severe ROP) and a marked decrease in the need for laser procedure (Unpublished data; figure 3).
Figure 2.Proportion of infants with BPD (Oxygen at 36 weeks) after implementing a practice guideline regarding oxygen administration and monitoring. (One center: CSMC and data from Vermont Oxford Network, large data base with > 25,000 infants < 1,500 gm).
Figure 3.Proportion of laser surgery in infants with birth weight 500-1,500 grams. The implementation of education and of a last generation SpO2 monitor (Masimo®) were associated with a marked decrease. (Single center, compared to VON data base).
In order to accomplish these changes in practice, it was necessary to "change culture". For this, we studied about the fascinating topic of culture change in health care. We learned that it is important to understand the meaning of organizational culture in NICU, since each facility has its own unique character that strongly influences the quality of care and overall environment. When managing culture change one can try to lead reform or transformation. We learned that coming to grips with culture in NICU facilities is a serious and necessary undertaking if real transformation is to occur. To implement transformation, every member of the leadership team, including medical directors, must be prepared for the hard work and time needed to shift organizational culture. A 'First Order Change' implies doing what you do better; by quantitative reproduction and repetition (i.e.: "Change OF culture"). The 'Second Order Change', on the other hand, is qualitative growth, something different. (i.e: "Change IN culture"). This is said to be necessary when an existing culture has begun to stagnate. We also learned that professional values, affirmed over years and decades, are resilient enough to frustrate many attempts to produce change. That is why the actions should not just attempt to modify familiar behavior.
One important point in this process was to remind all of us all of the time that, fairly frequently, in relation to oxygenation and complex physiologic changes, "more important than what we see is what we do not see". What we believe is that as useful as SpO2 monitors are, we should not fix ourselves with a SpO2 value (which we can see) and then chase it. Our approach is somehow different, learning and thinking about oxygen saturation curves, SpO2 monitors, their differences and the pitfalls of some, and the changes that may be induced on VGEF, IGF-1, and radical oxygen species by transient elevations of PaO2, all of which cannot see. Another important point in our own education was to accept that there has never been convincing evidence for the "rational" use of supplemental O2 in caring for preterm infants but there is physiological evidence and knowledge of the relation between SpO2 and Hemoglobin. The important concepts in which the educational process can be based and a change in culture be achieved are summarized in table 2.
Based on our findings we can conclude that adequate SpO2 monitoring equipment (Masimo Signal Extraction Technology®) accompanied by implementation of a strict clinical practice of oxygen administration and monitoring which avoids "high" SpO2 and minimize wide fluctuations from the time of birth and during the first few weeks of life are associated with a significant decrease in severe ROP. This is accompanied by no increase in mortality, no increase in developmental anomalies and a decrease in BPD. We also concluded that the intercenter (and within center) variability described for ROP rates is related at least in part to differences in clinical practices aSUB> administration and monitoring. We are NOT concluding that blindness can be completely eliminated by strict efforts to keep "FiO2 < 40 %" or that blindness can be completely eliminated by maintaining SpO2 at 85-93 %. Of course we are also not able to conclude which is the best SpO2 level for very preterm infants.
Avoiding these high saturations (97-100 %) in premature infants breathing (monitored) supplemental O2 is not the same as to recommend a set and fixed range of "lower O2 saturations". However, HIGH O2 saturation and HIGH FiO2 when not needed are practices with increased risk which can, and should, be avoided. The predicament of the clinician and bed side care provider is then which one of the ranges less than 95 % is safe and beneficial for different gestational and postnatal ages. This is not known and awaits further study. In our practice we chose a range as a target, with a span of about 9 % saturation points (85-93 %). We are not saying this is the way to handle all VLBW infants from birth until discharge if they are still breathing supplemental oxygen. In the tiniest of babies some speculate that maybe even lower SpO2 values (82 %-88 % or 91 %) may be sufficient, with the caveat that when an infant is not able to oxygenate with a possible diagnosis of pulmonary hypertension or when an infant gets older and has BPD or severe ROP these ranges of SpO2 are individually decided.
We do recommend education, and expanding the knowledge and understanding of bedside care providers of what is known to be risky. We consider that after an adequate process most would agree what are "bad practices" in this regard. Eradicating practices known to be associated with increased risks and changing a monitor with false readings and alarms and holding periods, could only be beneficial in clinical practice.
What we recommend then is to "change culture". This includes the following: a) monitor FiO2 and SpO2 all the time; b) increase our awareness that when infants breath FiO2 > 0.21 and the O2 saturation is > 95 % and up to 100 % the relation saturation/PaO2 may be lost; and therefore not to target high O2 saturations when FiO2 > 0.21 is being used. In addition we advocate: c) avoidance of cycling and wide, and rapid increases in FiO2, d) documentation; e) staying by the bedside when the infant is "recovered" after "an episode"; and f) to initiate weaning quickly but to wean slowly after this. In addition, we recommend using reliable last generation pulse oximetry monitoring to avoid responding to "false alarms"; and to maintain alarm limits as long as the infant is receiving FiO2 > 0.21 during high risk periods of development, starting from the time of birth. Finally, we recommend managing the infant with O2 saturations which, by known physiologic relations between hemoglobin and O2, will be related to a PaO2 which is "not low or high" by currently accepted standards. We must accept with humility the uncertainty of what the "best" or "ideal" saturation range is for all preterm infants of all gestational ages, at all postnatal ages. This needs further detailed study.
Finally, the worse incidence and progression of ROP seen in some regions or units makes one wonder if any of the many changes that have occurred in clinical neonatal care in the last 12-15 years may be partly responsible for this. Of the entire major therapeutic changes that have entered clinical practice, only one was well studied: exogenous surfactant. This practice is associated with more acute changes in oxygenation early on in life. Unfortunately, around the time of implementation of routine use of surfactant the TcPO2 monitors were changed to O2 saturation monitors. Some NICU's implemented "titration policies" (up and down) potentially associated with large PaO2 swings. Recently "higher" PaCO2's are being accepted and possibly more apneic episodes are tolerated since CPAP use is more prevalent. How do these combinations affect the developing retina is still not fully known.
Ongoing and Future studies
The AVIOX study (Actual Versus Intended OXygen saturation) was just presented in preliminary form at a research meeting92 in May 2004. This study shows that the proportion of SpO2 values within intended range varied between 16-71 % at different study centers. Most noncompliance was above the intended range, lending support to the fact that routine care of tiny premature infants who receive supplemental O2 varies substantially among different centers.
The POST (Pulse Oximetry Saturation Target) ROP trial is being planned extensively by Dr Cole and a large group of investigators. This will include tiny preterm infants. For this proposed randomized, blinded, controlled trial the target saturation ranges from the time of birth proposed are 85-89 % for one group and 91-95 % for the other. The results will hopefully help clinicians in better determining the safe and effective saturation to aim for. An editorial from December 2003 in Pediatrics1 advocating the POST ROP trial states that "Evidence of net benefit or harm from one outcome should be considered in the context of other major outcomes. For example, it would be inappropriate to terminate recruitment because of a 3 % reduction in severe ROP in the lower oxygen group before the trial had accumulated sufficient power to exclude a 6 % increase in mortality or severe neurodevelopmental impairment in the same group. In this case, if the trial were prematurely terminated and lower oxygen became the clinical standard, for every baby whose sight was saved, two would die or survive with major disability." In addition, other smaller trials and basic studies are starting to address new potentially useful drugs for decreasing abnormal retinal angiogenesis. They include exploring in a mouse model with neuropeptide Y expression, somatostatin analogs, ibuprofen and squalamine93-96 among others, to inhibit neonatal retinal neovascularization and stimulate regression of oxygen-induced retinopathy. This innovative work awaits further confirmation and may become of clinical value in the future.
Final Comments
Oxygen was entered into neonatal practice in the absence of any randomized studies. Furthermore, it has not been measured well and routinely, even today with SpO2 monitors. However, it is one of the drugs most frequently used in NICU's, many times without any limits or control. If we jump now to recommending "lower oxygen saturations" without a reliable controlled trial, we may be condemning more children to death or severe disability than we ever save from blindness. However, to avoid or eradicate proven bad practices is not simply to use "lower oxygen saturations". Currently, ROP is an unfortunate growing global problem, a persistent sight-threatening complication of ELGA infants (GA < 28 wks) in the industrialized nations and also of larger preterm infants in the developing nations. The visual outcome after severe ROP is unfavorable even if treated, and ROP is the most common cause of blindness.
The prevention of ROP may be possible by maintain a focus on research and also in clinical practice. Even though it is possible that in a not too distant future we may be able to use some preventive treatment, like drugs which modulate angiogenesis (VGEF or NPY receptor blockade, COX-2 inhibition, squalamine) or others like inositol, today we can change our culture and modify the relation between knowledge and the care the infants receive, changing clinical practice to eradicate 'bad practices'. We must not ignore the lessons of history, lest we are forced to repeat them, to the immense cost of these premature babies - the most vulnerable patients - and their families.
Our observations have shown us that in many cases, more important than what we see in routine practice may be what we do not see. This can be applied to what happens to many babies in delivery rooms or NICU's around the world in relation to oxygen dose, oxygenation and ROP.
"The essential is invisible to the eye", as Saint Exupery wrote in "The Little Prince".