Potentially painful invasive procedures are often performed for diagnostic or therapeutic purposes in hospitalised paediatric patients. Approaches, such as virtual reality (VR), should be sought in order to minimise pain and anxiety during these procedures.
Materials and methodsHospitalised patients between 4 and 15-years-old requiring an invasive procedure were included. Pain and anxiety evaluation scales were given to children, relatives and health workers. A comparison was made with patients in whom VR was used (with or without concomitant use of a prilocaine/lidocaine 2.5% analgesic cream) and patients in whom neither VR nor analgesic cream were used.
ResultsThe study included 58 patients, 38 in the VR group and 20 in the control group. Pain scores, as performed by patients, relatives and health workers, significantly decreased in the VR group (control group median 4/5 vs. VR group median 1/5, P<.001). Patient-reported anxiety scales were also lower in the VR group (control group median 4/5 vs. VR group 1/5, P=.001). The number of punctures (R2: 0.5, β: 0.6; P=.01) and the lack of analgesic techniques (β: −0.9; P=.02) were associated with higher scores in patient-reported pain scales.
CommentsThe use of VR can reduce pain and anxiety during invasive procedures in hospitalized children.
La realización de procedimientos invasivos dolorosos con fines terapéuticos o diagnósticos en pacientes pediátricos hospitalizados es frecuente en la práctica diaria. Se deben buscar estrategias encaminadas a disminuir el dolor y la ansiedad durante estas técnicas, como el uso de realidad virtual (RV).
Materiales y métodosSe realizó un estudio observacional, analítico y prospectivo, en el cual se incluyeron todos los pacientes pediátricos hospitalizados de entre 4 y 15 años que precisaron procedimientos invasivos. Se recogieron escalas de valoración de dolor y ansiedad a los pacientes (ajustadas a su edad), familiares y personal sanitario y se comparó el uso de la RV, de manera aislada y asociado a una crema anestésica (prilocaína/lidocaína crema 2,5%), con un grupo control en el que no se utilizó ninguna técnica analgésica.
ResultadosSe incluyeron 58 pacientes, de los cuales 38 usaron la RV (grupo RV) y 20 pacientes no recibieron ninguna técnica analgésica ni de distracción (grupo control). El uso de RV disminuyó las puntuaciones en la mediana de las escalas de dolor en niños, familiares y personal sanitario (grupo control 4/5 vs. grupo RV 1/5, p<0,001), y en las escalas de ansiedad en niños (grupo control 4/5 vs. grupo RV 1/5, p=0,001). En el análisis multivariante, el número de punciones (R2: 0,5; β: 0,6; p=0,01) y la ausencia de técnicas coadyuvantes (β: −0,9; p=0,02) se asociaron con puntuaciones más elevadas en la escala del dolor en niños.
ComentariosEl empleo de RV disminuye el dolor y la ansiedad durante la realización de procedimientos invasivos en pacientes pediátricos hospitalizados.
It is common for paediatric inpatients to undergo invasive procedures that may be painful during their hospital stay. For this reason, methods are being introduced to provide pain relief and reduce anxiety during such procedures.1 In fact, the Charter of the European Association for Children in Hospital stipulates the right of every child to be protected from unnecessary medical treatment and physical or emotional distress.2
These methods can be divided into pharmacological and nonpharmacological interventions. Nonpharmacological interventions can be further subdivided into support methods (such as viewing videos, reading books and companionship), cognitive methods (such as relaxation or distraction) and physical methods (positioning, massaging and stimulation of the skin by means of vibrations or the application of heat or cold).1,3
As regards the cognitive methods, it has been hypothesised that the “ideal distractor” is one that draws attention to something that involves the use of several senses (vision, touch, hearing) and has an emotional impact, so that it can compete with the unpleasant stimulus. Few articles in the literature have addressed the use of emerging technologies, such as virtual reality (VR), for analgesia in the paediatric population during interventions such as burn care, chemotherapy, dental procedures and other medical procedures.4–9 Neurophysiological studies that used functional magnetic resonance imaging have also found that it achieves a reduction in perceived pain.10,11
When it comes to pharmacological methods, the use of topical anaesthetic drugs avoids systemic effects and reduces adverse events. In clinical practice, a cream consisting of a combination of prilocaine and lidocaine (both at 2.5%) is frequently used and has proven effective as an anaesthetic in minor invasive procedures.12–18
The aim of the study was to assess whether the use of VR, both in isolation or combined with the use of a lidocaine/prilocaine cream, reduced pain and anxiety during invasive procedures in patients hospitalised in the paediatric ward without producing clinically relevant adverse effects.
Materials and methodsStudy design and sampleWe conducted an observational, analytical prospective cohort study in which we included patients aged 4–15 years admitted to the paediatric ward between November 2016 and June 2018 that needed to undergo an invasive procedure.
Inclusion and exclusion criteriaWe included patients aged more than 4 years to be able to use VR and validated scales. We excluded patients who were clinically unstable, received other types of analgesia during the procedure, with cognitive impairments or in whom it was not possible to use the scales.
Since we conducted an observational study, decisions on which methods were to be used for analgesia were made by the provider that ordered the invasive procedure in adherence with the routine clinical protocols of the unit. We considered that allocating patients randomly to a control group would be unethical, since we had available methods for pain relief whose efficacy had already been demonstrated in past research. Thus, the patients that constituted the control group were those who required the interventions in evening and night shifts, as the protocol had not been introduced in those shifts yet.
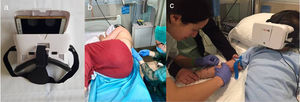
MaterialsWe delivered the VR intervention with the Woxter Neo VR1 glasses (adjusting the lenses to match the interpupillary distance) fitted with a mobile device (Fig. 1a) and headphones to enhance the immersion in the virtual world (Fig. 1b and c). We allowed patients to choose from a variety of previously reviewed videos based on the age and tastes of the patient.
Some patients received a prilocaine/lidocaine cream as adjuvant treatment as established in our analgesia protocol, which includes the following recommendations from the manufacturer: apply 1–2g of cream in the area where numbness may be needed (only on intact skin) over an area not exceeding 10cm2 and cover with an occlusive dressing, let sit for 60min and puncture the skin within 30min from removing the dressing.
Study variablesWe recorded data for the following variables: age, sex, diagnosis, type of procedure, adjuvant use of topical analgesic cream or lack thereof, adverse events, pain and anxiety scale scores self-reported by patients (Wong-Baker FACES pain rating scale [WBS] in patients aged 4–6 years, rated on a scale of 0–5,19,20 Visual Analogue Scale [VAS] in patients aged more than 7 years, rated on a scale from 0 to 5,19,21 and Children's Fear Scale [CFS], rated on a scale from 0 to 4).1,22 We also documented proxy reports of pain and anxiety given by adults that were present during the procedure, including family members and health care staff, who completed the scales independently from the child: numeric rating scale (NRS), on a scale of 0–5,19,23 and CFS for assessment of anxiety, on a scale of 0–4. In every pain assessment scale used, 0 stood for the absence of pain and 5 for the most severe pain imaginable. We modified all the scales to have scores ranging from 0 to 5 in order to make the results comparable (Appendix A). We also conducted a satisfaction survey. When it came to the use of topical analgesic cream as adjuvant treatment, we documented whether its use conformed to the specifications given in the summary of product characteristics and any adverse events, as well as the methaemoglobin level in patients in whom a blood gas analysis had been performed.
The study was approved by the Ethics Committee of the hospital (CEIC 295/17). We informed all patients and their guardians about the study, and obtained their informed consent in writing for participation in the study.
We performed the statistical analysis with the software SPSS Statistics version 21 (IBM SPSS Statistics; Armonk, NY, USA). We have summarised qualitative variables data as percentages and quantitative data as median and interquartile range (IQR). We compared qualitative variables by means of the chi square and Fisher exact tests. We compared continuous quantitative data with the Mann–Whitney U and Kruskal–Wallis tests. We assessed the correlation between variables using the Spearman's correlation coefficient. We performed a multivariate analysis and fitted a linear regression model in which the dependent variable was the pain assessment scale score and the covariates were the age, sex, type of procedure, use of adjuvant interventions and number of punctures performed during the procedure. Statistical significance was defined as a P-value of less than 0.05 in any of the tests.
ResultsSample characteristicsThe sample included 58 patients with a median age of 120 months (IQR, 84–156); 34 participants (58.2%) were female.
The most frequent reason for admission was acute gastrointestinal illness followed by acute respiratory illness. As for the type of procedure, most of the patients required extraction of a blood sample (Table 1).
Reason for admission and type of procedure performed.
| Reason for admission | n (%) |
|---|---|
| Gastrointestinal illness | 24 (41.4) |
| Respiratory illness | 7 (12.1) |
| Neurologic illness | 5 (8.6) |
| Infectious illness | 4 (6.9) |
| Other | 18 (31) |
| Type of painful procedure performed | n (%) |
|---|---|
| Blood draw | 49 (84.5) |
| Peripheral venous catheter placement | 8 (13.8) |
| Lumbar puncture | 1 (1.7) |
Virtual reality was used as a distraction technique in 38 patients (VR group), and 20 patients in whom no analgesia or distraction techniques were used constituted the control group. Both groups were comparable in terms of the age, sex, number of punctures and types of procedures performed (Table 2).
Comparison of control group and virtual reality group.
| Control group (n=20) | VR group (n=38) | P | |
|---|---|---|---|
| Group characteristics | |||
| Age in months (IQR) | 138 (90–177) | 108 (72–144) | .06 |
| Sex, n (%) | .84 | ||
| Male | 8 (40) | 16 (42.9) | |
| Female | 12 (60) | 22 (57.1) | |
| Number of punctures, median (IQR) | 1 (1–2) | 1 (1–2) | .41 |
| Results of pain and anxiety scales, median (IQR) | |||
| Pain reported by patient | 4.0 (3.0–4.0) | 1.0 (0–1.25) | <.001* |
| Anxiety reported by patient | 4.0 (3.0–4.0) | 1.0 (0–1.0) | .001* |
| Pain reported by parents | 4.0 (3.0–4.0) | 1.0 (1.0–2.0) | <.001* |
| Anxiety reported by parents | 3.0 (2.5–3.5) | 1.0 (0–1.0) | <.001* |
| Pain reported by health providers | 4.0 (3.0–4.0) | 1.0 (1.0–2.0) | <.001* |
| Anxiety reported by health providers | 3.0 (1.5–3.0) | 1.0 (0–1.0) | <.001* |
IQR, interquartile range; VR, virtual reality.
When we compared the scores of the assessment scales, we found lower scores for both pain and anxiety as reported by patients, family members and health professionals in the VR group, differences that were statistically significant (P<.001) (Table 2).
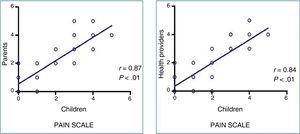
None of the patients experienced adverse events associated with the use of VR. We found a strong correlation between the pain perceived by patients and the pain reported by family members (r=0.87; P<.01) and the health care staff (r=0.84; P<.01) (Figure 2), as well as a good correlation between the anxiety perceived by patients and the anxiety reported by family members (r=0.782; P<.01) and the health care staff (r=0.738; P<.01).
The linear regression analysis showed that the number of punctures (R2=0.5; β=0.6; P=.01) and the lack of use of adjuvant interventions (R2=0.5; β=−0.9; P=.02) were associated with higher scores in pain scales in children.
Analysis of the combined use of topical analgesic creamTopical prilocaine/lidocaine cream was used in combination with VR in 13 patients (VR+analgesic cream subgroup), and we compared this subgroup with the 25 patients in whom VR was used in isolation (VR without cream) and with a control group that was comparable in terms of age, sex, number of punctures and types of procedures (Table 3).
Comparison of control group, virtual reality and no cream group and virtual reality combined with analgesic cream group.
| Control group (n=18) | VR and no cream group (n=16) | VR+analgesic cream group (n=22) | P | |
|---|---|---|---|---|
| Group characteristics | ||||
| Age in months (IQR) | 132 (81–159) | 113 (78–150) | 84 (66–114) | .06 |
| Sex, n (%) | .50 | |||
| Male | 7 (38.9) | 7 (45.5) | 8 (38.5) | |
| Female | 11 (61.1) | 9 (54.5) | 14 (61.5) | |
| Number of punctures, median (IQR) | 1 (1–1.25) | 1 (1–1) | 1 (1–1) | .79 |
| Serummethaemoglobin level (%), median (IQR) | 1 (0–1) | 0.75 (0–1) | 0.35 (0–1) | .46 |
| Results of pain and anxiety scales, median (IQR) | ||||
| Pain reported by patient | 4.0 (3.0–4.0) | 1 (0.5–2) | 0 (0–1) | <.001* |
| Anxiety reported by patient | 3.5 (3.0–4.75) | 0.5 (0–1) | 0.5 (0–1) | .009* |
| Pain reported by parents | 4.0 (3.0–4.0) | 2 (1–2) | 1 (0–2) | <.001* |
| Anxiety reported by parents | 3.0 (2.25–3.75) | 1 (0–1.5) | 1 (0–1) | <.001* |
| Pain reported by health providers | 4.0 (3.0–4.0) | 2 (1–2) | 0 (0–1) | <.001* |
| Anxiety reported by health providers | 3.0 (2–3.0) | 1 (0–1) | 0 (0–1) | <.001* |
We compared quantitative variables by means of the Kruskal–Wallis test and categorical variables by means of the Fisher exact test or chi square test. The data are summarised as median and interquartile range or as percentages.
IQR, interquartile range; VR, virtual reality.
In comparison with the control group, the scores in the pain and anxiety assessment scales as reported by patients, family members and health care providers were significantly lower in both VR subgroups (P<.05) (Table 3).
When we compared the VR without cream and the VR+analgesic cream subgroups, we found lower pain scores in the VR+analgesic cream subgroup as reported by the patients (P=.02), the parents (P=.03) and the health care staff (P=.001). When it came to the anxiety measures, we only found lower scores in the reports made by health providers (P=.03), and no differences in the reports made by the patients (P=.86) or the parents (P=.19).
None of the patients experienced adverse events or had elevated methaemoglobin levels associated with the use of analgesic cream, and we found no differences in these parameters between groups (Table 3).
The analgesic cream was used as directed in 100% of cases, with the cream being allowed to sit under the dressing for a median of 60min (IQR, 60-60) and performance of the procedure within 2min of its removal (IQR, 2–5). We found no correlation between the pain reported by the patient and the duration of exposure to the cream (r=0.408; P=.24) or the time elapsed from removal of the cream to performance of the procedure (r=0.16; P=.64).
Satisfaction survey and characteristics of previous invasive proceduresWe conducted a satisfaction survey with participation of 54 patients, 58 family members and 50 health providers. Eighty-five percent of patients had previously required invasive procedures, but only 4.3% reported the use of analgesia during that prior experience, which in all cases consisted on the application of a topical cream.
In the patient survey, 85.7% reported perceiving less pain than expected with the analgesic methods used, and 63.4%, feeling less anxious. In addition, 95.5% expressed the wish that these techniques be used again should they require additional procedures in the future.
In the survey of family members, 74.1% reported that they perceived less pain in the patient and 31.5% that they perceived less anxiety; 96.3% requested the use of the same techniques if the patient required procedures in the future. Also, 86.8% believed that the use of these techniques had facilitated performance of the procedure, and 84.6% that they themselves experienced less anxiety during the procedure.
The survey of health professionals showed that 92% considered that the use of analgesic interventions had facilitated performance of the procedure.
DiscussionThe findings of our study suggest that the use of adjuvant interventions during invasive procedures can reduce pain and anxiety in patients hospitalised in the paediatric ward, with results similar to those reported in the previous literature with the use of VR in isolation5–9 or combined with topical analgesic creams.12–17
Although most patients in our cohort were previously healthy, a high proportion had needed invasive procedures in the past. Previous studies suggest that the analgesic effect of distraction techniques may be greater in patients with underlying disease,6,9 a population in which it is possible to compare the severity of perceived pain with the pain perceived during previous experiences, but in our study we also found evidence of this effect in previously healthy patients admitted to hospital due to acute disease.24
One of the strengths of our study is that patients were able to choose from several videos that had been previously reviewed, as past evidence suggests that having choices achieves a greater reduction of pain.9
In most of the previous literature, the pain scales were administered only to the patients, unlike in our study, where we also obtained proxy reports from family members and health care staff. We administered the scales individually and in writing to avoid influencing the responses of participants, and we found a very strong correlation between the self-reports of patients and the assessments of the pain experienced by patients made by family members and the health care staff. The assessment of anxiety in children is an area that still needs further development, as there are no validated scales for measuring anxiety in children aged less than 5 years. In this study we used the CFS in children aged more than 4 years and obtained results that were similar to those reported in the previous literature, where this scale was used in children aged more than 5 years.22
The findings of our study are consistent with those of other studies that have demonstrated that VR is a nonpharmacological cognitive method that is quite helpful for relief of pain and anxiety. There are previous studies with very different characteristics that support the use of this technique conducted in healthy adults,25,26 healthy children exposed to pain through the cold pressor test,27 paediatric cancer patients undergoing phlebotomy procedures,6 paediatric cancer patients undergoing lumbar puncture,7 adults and children receiving care for burns,28,29 paediatric outpatients undergoing phlebotomy procecures,5 etc. To our knowledge, ours is the first study conducted in patients hospitalised in a paediatric ward, a subpopulation that has not yet been assessed in the literature that could unquestionably benefit from these modalities of analgesia.
Some authors assert that the use of active distraction, where the patient is immersed in the film, is more effective compared to passive distraction through videos,30 a hypothesis supported by our findings, and the isolation created by the glasses and headphones facilitates detachment from the unpleasant situation to which the patient is exposed.
None of the patients experienced adverse events associated with the use of VR, which may include nausea, dizziness or other motion sickness symptoms described in previous studies.31 This aspect may be associated with the choice of VR device. We ought to highlight the differences between VR glasses used in combination with mobile devices, which are safe to use from age 4 to 5 years if they allow adjustment of the interpupillary distance, and standalone headsets (with a 3D screen) designed for gaming, which are not recommended for children aged less than 12 years since they are the interface that usually produces the aforementioned side effects.
There is controversy in the literature regarding the use of analgesic creams in invasive procedures, although most of the research has assessed their use in isolation.32,33. When it comes to analgesic creams as adjuvant treatment, as was the case in our study, most studies support their use.12–17 Some studies have not found any benefit from their use, which could be due to the simultaneous use of distraction, which may mask the effects of creams.34 Our study found an increase in pain relief when cream was used in combination with VR, although it did not add to the anxiety-reducing effect of VR. Although some authors have reported adverse events associated with the use of topical creams (methaemoglobinaemia, seizures, vasoconstriction and contact dermatitis),18 we had no such case in our sample. This is probably due to the fact that the directions for the use of this drug were always adhered to, in contrast to some of the cases described in the literature in which topical creams were used inappropriately, for instance, by applying them to a large surface or on broken skin.35–37 Some authors have reported that these creams may cause vasoconstriction, which can be counteracted with the application of heat,38 while others state that their application does not interfere with the performance of the procedure.39 Our data support the latter view, as we did not find any differences between the groups in the number of punctures, a variable whose increase may indicate increased difficulty in performing a procedure.
We found a high degree of satisfaction in the patients and their family members in relation to the use of VR, as they considered that in addition to alleviating pain and anxiety, it facilitated the performance of the procedure and also reduced the anxiety of the accompanying person. Most patients and their families requested the use of VR if they happened to need further procedures in the future.
As for the limitations of this study, the chief one is its observational design, as patients were not randomised to different groups, although comparing the baseline characteristics of patients in each group we found that the 2 groups were comparable.
Another limitation is the low number of patients in the sample that underwent other interventions commonly performed during hospitalization, such as lumbar puncture. This is due to the fact that since these interventions are known to be more painful in and of themselves, their performance involved the use of drugs or other analgesic measures that resulted in the exclusion of these patients from the study.
Last of all, it is important to highlight that the main outcome variables in our study were clinical scales that cannot but be somewhat subjective, although the use of other parameters, such as blood pressure and heart rate, complicated their interpretation, as the patients in the study had acute illness and could have fever or breathing difficulties at the time the procedure was performed, which was a potential a source of bias. To minimise the impact of this limitation, we used these instruments to assess the perception of not only the patients, but also the family members and the health care staff.
We expect that future performance of randomised clinical trials in paediatric patient comparing the use of VR and other interventions such as analgesic cream alone or the application of cold, heat or vibration during painful procedures will provide us with more rigorous evidence on these techniques.
CommentsThe use of VR during invasive procedures in patients hospitalised in the paediatric ward reduced the pain and anxiety perceived by the patient, families and health care staff. We found that pain scores increased as the number of punctures used in a procedure increased and decreased with the use of adjuvant interventions. We did not identify any adverse events associated to the use of VR or analgesic cream or any data suggesting that they interfered with performance of the procedure.
Patients and their families expressed a high level of satisfaction in relation to the use of VR, as they found that in addition to reducing pain and anxiety, it facilitated performance of the procedure and also reduced the anxiety of the accompanying person, and stated they would request the use of VR should they need additional procedures in the future.
Conflicts of interestThe authors have no conflicts of interest to declare
Please cite this article as: Toledo del Castillo B, Pérez Torres JA, Morente Sánchez L, Escobar Castellanos M, Escobar Fernández L, González Sánchez MI, et al. Disminuyendo el dolor en los procedimientos invasivos durante la hospitalización pediátrica: ¿ficción, realidad o realidad virtual?. An Pediatr (Barc). 2019;91:80–87.
Previous presentation: This study was presented as an oral communication under the title “Dolor Zero” en planta de hospitalización pediátrica: ¿ficción, realidad… o realidad virtual? At the 66 Congress of the Asociación Española de Pediatría; June 7–9, 2018; Zaragoza, Spain.