The use of infant formulas (IFs) in health care settings carries an intrinsic risk of contamination during the preparation of bottles.
To assess the current situation in Spain, we conducted an online survey during March 2024 through the mailing list of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (SEGHNP, Spanish Society of Pediatric Gastroenterology, Hepatology and Nutrition). We used a Google Docs questionnaire that included eight closed-ended questions. The survey did not explore any aspects other than formula reconstitution, such as critical control points. We mailed the questionnaire to 155 hospitals that offered obstetric care in Spain and received responses from 56 (36.1%); of this total, 23.6% managed fewer than 1000 births a year, 54.5% managed 1000–3000 births a year and 21.8% more than 3000 births a year. We received responses from at least one center in each autonomous community in Spain, with the exception of La Rioja and Extremadura, with the highest frequency corresponding to Madrid (14% of total responses).
The highest safety1,2 is achieved with the use of liquid ready-to-feed formulas for healthy infants, which were used by 94.6% of participating hospitals. Formulas for which this presentation is not available (hydrolyzed, elemental, metabolic) have to be reconstituted from powder, which entails a series of risks.
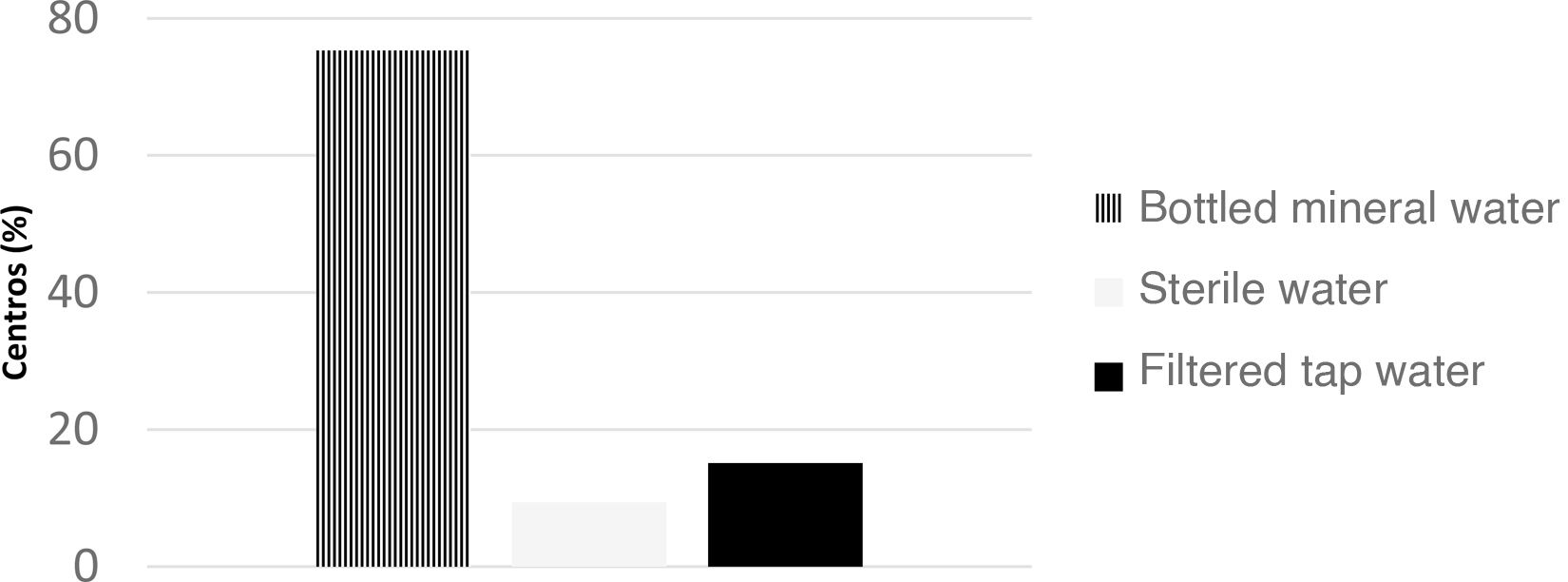
There is risk associated with the use of potentially contaminated water. Different guidelines recommend using “bottled” or, more specifically, “sterile” water1,3 or tap water purified with filters capable of removing substances smaller than one micron. Current Spanish law dictates that mineral water must be free of any microbial pathogens. Based on our survey, bottled mineral water is used most frequently for IF reconstitution in Spain (Fig. 1).
Another important factor in potential contamination is the location of formula preparation. According to the results of our survey, 17.8% of centers do not have a dedicated, physically separated space for the purpose, and 3.6% only have such as space in the neonatal intensive care unit. In these centers, IF is prepared in the staff station of the ward. Published guidelines1–3 recommend setting up a specific, dedicated and equipped space separate from patient care areas for powdered IF handling and reconstitution.
Even if staff adheres strictly to the directions of the manufacturer, powdered IFs are not sterile products and therefore can contain pathogens.2 Thus, there is a risk associated with the potential contamination of the formula, especially of contamination by Cronobacter sakazakii (CS), whose source is not the water or location used for preparation, but the powdered formula itself.4
Cronobacter sakazakii causes invasive disease in infants, with a fatality rate of 20%–50% that is highest among preterm infants. The main transmission vehicle for CS infection is powdered IF, with the literature reporting a clear association with these products in 50%–80% of cases.4,5 Infections by CS have been described since the 1960s (although they are believed to be significantly underdiagnosed), and there are repeated outbreaks involving this organism, the last one reported in the United States in 2022, which resulted in the death of two children.4
To minimize the risk of CS infection, it is recommended that formula be reconstituted with water heated to a minimum temperature of 70 °C followed by rapid cooling to less than 4 °C, in addition to keeping formula refrigerated at a temperature not exceeding 5 °C from the moment it is reconstituted until it is consumed.1,5
In many cases, on account of the relatively low frequency of CS infections, the relatively high risk of burns associated with heating and the resulting nutritional modification of the formula (especially as regards probiotics), this recommendation is not given for home preparation if the bottle is to be administered immediately after. Several authors and organizations in Spain, including the Agencia Española de Seguridad Alimentaria y Nutrición (Spanish Food Safety and Nutrition Agency),6 recommend this practice for children at risk (newborns, preterm or low birth weight infants, children with immune disorders).5
When it comes to the preparation of IF in health care settings, some organizations, such as the British Dietetic Association, recommend heating the water above 70 °C and reconstituting the formula at this temperature, while others, like the Academy of Nutrition and Dietetics of the United States, do not recommend preparation with hot water, although the latter recommendation preceded the latest outbreaks reported in the country, where CS was identified in IF containers that had not yet been handled.
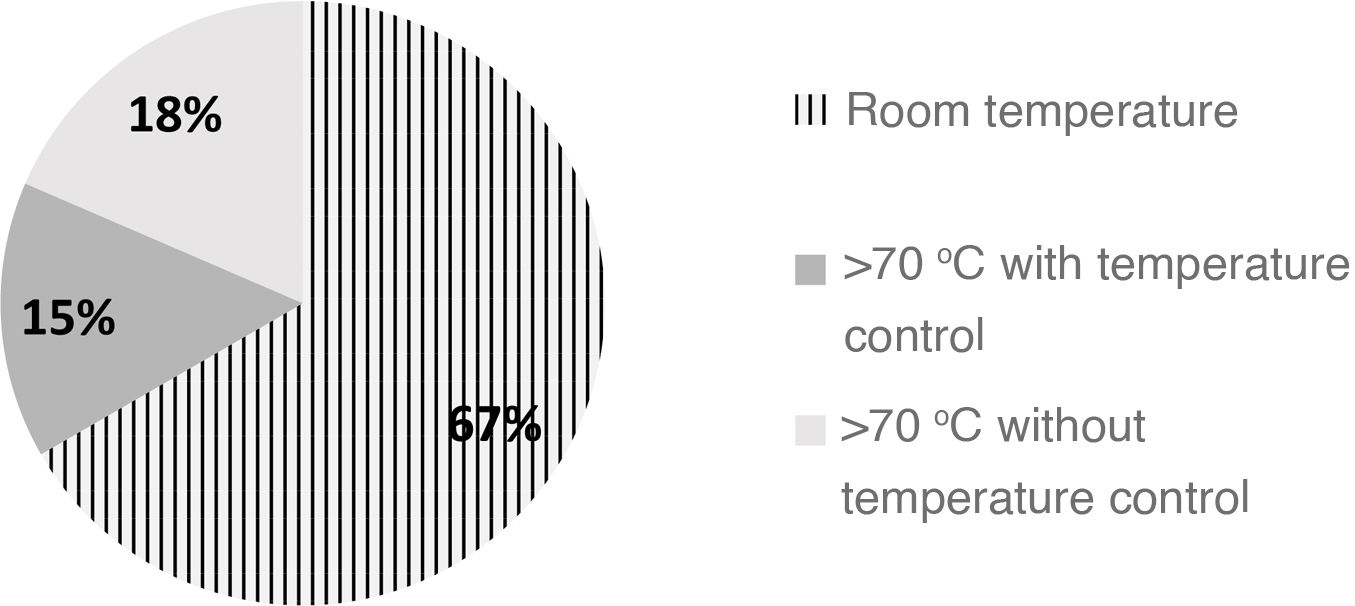
In Spain, based on the findings of our survey, approximately 70% of hospitals do not reconstitute formula with water heated above 70 °C (Fig. 2) and 37% do not keep reconstituted IF at a temperature of less than 5 °C, in most cases because the formula is administered immediately after preparation.
A consensus-based protocol for health care facilities needs to be developed to pursue the optimal risk-benefit balance.