The objective of this document is to review the current recommendations in the management of the foetus and the newborn child born to mothers with autoimmune thyroid disease. In 2017, the American Thyroid Association published guidelines for the diagnosis and management of thyroid disease during pregnancy and post-partum. In this guide, 97 recommendations were made, and an algorithm for the diagnosis and treatment of gestational hypothyroidism was proposed. Also, in the last year, a wide review was published on the foetal and neonatal approach of the child of a mother with Graves’ disease. The importance of the determination of maternal antibodies against thyrotropin receptor in the second half of pregnancy is stressed, in order to adequately stratify the risk in the neonate.
El objetivo de este documento es revisar las recomendaciones actuales en el manejo del hijo de madre con patología autoinmune tiroidea. En este 2017 se ha publicado la guía de la Asociación Americana de Tiroides para el diagnóstico y manejo de la enfermedad tiroidea durante el embarazo y el posparto. En dicha guía se establecen 97 recomendaciones y se propone un algoritmo de diagnóstico y tratamiento del hipotiroidismo gestacional. También en este último año se ha publicado una amplia revisión sobre el abordaje foetal y neonatal del hijo de madre con enfermedad de Graves. Se insiste en la trascendencia de la determinación de anticuerpos maternos frente al receptor de TSH en la segunda mitad del embarazo para estratificar adecuadamente el riesgo en el neonato.
In the last few years, there have been significant advances in our knowledge of thyroid disorders during pregnancy, which have resulted in the recent publication of clinical practice guidelines by the American Thyroid Association (ATA),1 European Thyroid Association (ETA)2 and the Sociedad Española de Endocrinología y Nutrición (Spanish Society of Endocrinology and Nutrition, SEEN).3 Since 2012, the ATA, the ETA and the Working Group on Iodine Deficiency and Thyroid Dysfunction of the SEEN recommend screening for thyroid dysfunction in nearly all pregnant women.1–3 This recommendation has led to an increase in the detection of thyroid disorders during pregnancy, which in turn has resulted in an increase in the treatment and followup of pregnant women and their newborns.
The development of healthy thyroid function in the foetus requires normal embryogenesis, differentiation and maturation of the thyroid gland, and also the integrity of the hypothalamic–pituitary–thyroid axis and the mechanisms that regulate the metabolism of thyroid hormones. The normal unfolding of all these processes depends on multiple foetal and maternal factors, with maternal thyroid function, thyroid autoantibodies and iodine intake playing key roles.
During pregnancy, the passage of thyroxine (T4) from the mother to the foetus protects the developing brain. Even in the early stages of pregnancy, T4 can be found in embryonal fluids, and later in pregnancy, when hormone secretion by the foetal thyroid has already started, maternal hormones continue to contribute to neurologic development.4–6
In areas where severe iodine deficiency is endemic, women may not have sufficient iodine stores and already have hypothyroxinaemia in the critical early stage of foetal neurodevelopment. Women with adequate iodine intake before and during pregnancy have sufficient intrathyroidal iodine stores and can adapt to the increasing requirements of pregnancy without difficulty. Although there is evidence that iodine supplementation may trigger thyroid autoimmunity in a small percentage of women, ensuring adequate iodine intake in pregnant women is a health priority. At present, a daily iodine intake of 250μg is recommended in all pregnant women, excepting those with hyperthyroidism or currently in treatment with levothyroxine.7
When we speak of maternal thyroid autoimmunity, we refer to the detection of thyroid autoantibodies in the pregnant women. In women of childbearing age, the prevalence of anti-thyroid peroxidase antibodies (TPOAb) and/or anti-thyroglobulin antibodies (TgAb) ranges between 8 and 14% depending on the study. Its presence in pregnant women is associated with an increased risk of maternal hypothyroidism during pregnancy.4,8–10 There are multiple studies in the literature on their presence and analysing the impact on the thyroid function of the foetus and newborn. Both TPOAb and TgAb of the IgG type can freely cross the placental barrier, which explains why more than 95% of newborns of mothers with Hashimoto thyroiditis (HT) have circulating autoantibodies.10
The prevalence of maternal hyperthyroidism due to Graves disease during pregnancy is much lower, ranging between 0.1 and 2.7%. The long-acting thyroid-stimulating hormone (TSH) receptor antibodies (TRAb) that cause this disease freely cross the placenta in the second half of pregnancy and have been shown to cause transient neonatal Graves disease in up to 2–20% of the offspring in cohort studies.
Infants born to mothers with HTThe most prevalent thyroid disorder in women of childbearing age is autoimmune thyroiditis due to Hashimoto disease, also known as Hashimoto thyroiditis (prevalence, 2.5%).
Impact on the mother and foetusThe presence of TPOAb and/or TgAb during pregnancy is independently associated with fertility problems, recurrent miscarriages, preterm birth and gestational diabetes.11–13 Some authors have reported an adverse impact on neuropsychological outcomes14 and an increased incidence of hearing loss and attention deficit hyperactivity disorder (ADHD)15–20 in children born to mothers that test positive for thyroid autoantibodies. These abnormalities could be directly mediated by autoantibodies even in the absence of concomitant maternal hypothyroidism, although the exact pathophysiological mechanism remains unclear.
In recent years, there have been initiatives for the establishment of routine screening for thyroid dysfunction and autoimmunity aimed at verifying normal thyroid function from the early stages of gestation. Establishing normal ranges for thyroid function markers during pregnancy poses challenges on account of the numerous adaptive changes that take place in this stage of life. In the first 10 weeks of gestation, TSH levels decrease due to an increase in the levels of β-human chorionic gonadotropin (βhCG) (thyrotropic effect) and placental type 3 deiodinase; later on, as βhCG levels decrease, levels of TSH increase as levels of FT4 decrease.
The most recent guidelines recommend early screening of thyroid dysfunction in pregnant women, starting with measurement of TSH levels. Treatment with levothyroxine is indicated when TSH levels are 10mU/L or greater or when TSH levels between 2.5 and 10mU/L are associated with low FT4 levels. The diagnosis of subclinical gestational hypothyroidism should be considered in women with TSH levels above the upper reference limit for the specific trimester of pregnancy. To this end, establishment of population- and trimester-specific reference ranges is recommended, which should be based exclusively on the values found in pregnant women with adequate iodine intake who have tested negative for TPOAb.1–4,21,22 If reference ranges are not available for the population of interest, it is recommended that a TSH upper reference limit of 4mU/L is used during pregnancy.1 When it comes to establishing a cut-off point to define low levels of thyroxine in pregnant women, many authors consider that measurement of total TT4 is more appropriate, given the greater variability of FT4 levels. Thus, the ETA recommends estimating the reference range for pregnancy by multiplying the applicable range in the general population by 1.5 (it is estimated that normal ranges of TT4 during pregnancy are about 50% greater compared to the general population).2
Impact on newbornsA small percentage of pregnant women with HT will have TSH-stimulation blocking antibodies (TSBAb), and an even smaller percentage will have thyroid-stimulating antibodies (TSAb). It used to be believed that these antibodies caused transient neonatal hypothyroidism. However, recent studies suggest that TPOAb and TgAb do not block the child's thyroid function.23–25
The current data show that approximately 25% of newborns of mothers with HT will have mild elevation of TSH or even subclinical hypothyroidism. Specifically, more than 75% have mild hyperthyrotropinaemia (TSH<15μIU/mL) on day 3 post birth, which resolves within a few days or weeks (levels normalise in >70% shortly before 1 month post birth), with spontaneous resolution in most patients.23,24,26,27 The development of hyperthyroidism in the newborn associated with the presence of TSBAb from a mother with HT is extremely rare (only one case reported in the literature). Therefore, it does not seem that the thyroid function of the foetus or the newborn could be permanently impaired by the presence of thyroid antibodies.
Management of infants born to mothers with HTStudies published in recent years have recommended followup of these children with protocols that require collection of multiple blood samples and several visits to a hospital.28 Currently, the benefits observed with such a followup hardly justify it, and based on our experience and the review of the recent literature we would not recommend it.23–27
In newborns of mothers with HT, screening for congenital hypothyroiditis at 48h post birth a non-invasive and sufficient intervention to assess thyroid function in the newborn.
The presence of hypothyroidism in pregnant women does not warrant a different approach to neonatal screening for thyroid dysfunction.
Infants born to mothers with Graves-Basedow diseaseChildren born to mothers with Graves disease (GD) are at significant risk of morbidity and mortality and thus require the use of appropriate protocols for their identification and management.28 The most recent guidelines and systematic reviews have offered suggestions on how to approach these patients, acknowledging that the lack of evidence and consensus precludes making specific recommendations.29
The antibodies that cause GD are long-acting TRAb, that belong to the immunoglobulin G class and can freely cross the placenta in the second trimester of gestation,2,29 of which there are two types:
- -
TSH-receptor stimulating antibodies that bind the TSH receptor in thyroid follicular cells and cause autonomous thyroid hormone production.
- -
TSBAb that bind the TSH receptor but do not trigger the intracellular signal cascade.
At present, two methods are available for the measurement of TRAb. Second-generation methods measure immunoglobulin levels and do not discriminate between thyroid-stimulating or thyroid-blocking antibodies, but they are inexpensive and widely available. Third-generation methods are less available, more complicated and more expensive, but can discriminate between blocking and stimulating antibodies. The presence of stimulating antibodies is associated with a high risk of neonatal hyperthyroidism.29
A mother with current GD or a history of treated GD may have a healthy child, a child with foetal and/or neonatal hyperthyroidism or a child with hypothyroidism.30 The coexistence of blocking and stimulating antibodies may delay the onset of hyperthyroidism.30–33
The levels of TRAb in a woman with GD may remain elevated for many years after its cure, be it by means of thyroidectomy, antithyroid medication or radioactive iodine. They should be measured between weeks 20 and 24 of gestation6; if the results are negative, the newborn can be considered to be at low risk, but if they are positive, the newborn should be considered to be at high risk.2,29,35
Impact on the foetus- •
Foetal hyperthyroidism:
It usually develops in the third trimester of gestation. It manifests with tachycardia, heart failure, non-immune hydrops fetalis, goitre, intrauterine growth restriction, preterm birth, accelerated bone maturation and craniosynostosis. These symptomatic cases may be treated with administration of antithyroid medication to the pregnant patient.2,32
- •
Risk of congenital malformations: secondary to treatment with antithyroid medication.
- ∘
Methimazole (MMI)/carbimazole: may produce aplasia cutis, embryopathy with facial dysmorphism, choanal atresia, oesophageal atresia, abdominal wall defects, umbilicocele, urinary tract defects and ventricular septal defects, especially if exposure occurs in the first trimester of gestation.2,33
- ∘
Propylthiouracil (PTU) can not only lead to maternal hepatotoxicity, but may also cause minor congenital malformations, such as cysts in the face and neck or urinary tract anomalies.2,33,36
- ∘
The ATA recommends suspending treatment during the first trimester if the mother with GD is stable in early pregnancy. If this were not possible, it is preferable to use PTU during this trimester and, given the risk of liver failure, switch to MMI in the second and third trimesters.2,33 The foetus is more sensitive to antithyroid drugs than the mother, so the mother should be given the lowest possible dose to maintain her hormone levels slightly above the normal range.2,6 Women with hyperthyroidism require a meticulous endocrinological followup, including measurement of TRAb levels and sonographic assessment of the foetal thyroid in the third trimester.2,29
- ∘
PTU and MMI are secreted in breast milk in very small concentrations, do not affect the thyroid of the newborn and are safe drugs during lactation.2
- ∘
- •
Neonatal hyperthyroidism or GD
There is considerable variation in the signs and symptoms of neonatal GD, and they may last 3–4 months, or even longer. Possible findings include goitre, tracheal compression, low weight, periorbital oedema, retraction of the eyelid, hyperthermia, diarrhoea, irritability, skin redness and warmth, difficulty feeding, stagnant weight gain, tachycardia, heart failure, hypertension, splenomegaly, cholestasis, thrombocytopenia and hyperviscosity. These symptoms are non-specific and could be attributed to congenital infection or sepsis. If these newborns are not identified and managed correctly, the mortality can be as high as 20%.2,29,35
Complications in the child are more severe if the mother remains hyperthyroid in the second half of pregnancy, as sustained normal levels of thyroid hormones are essential for the healthy development of the foetal central nervous system. Children of mothers with GD that are euthyroid at birth usually have a normal cognitive development, but children that have neonatal hyperthyroidism may experience neurodevelopmental impairment.33
- •
Neonatal hypothyroidism
Infants born to mothers with GD may also present features of hypothyroidism.32,34
- -
Transient central hypothyroidism. If maternal hyperthyroidism is poorly controlled during pregnancy, the high levels of thyroid hormones hinder the normal development and maturation of the thyroid gland and the hypothalamic–pituitary–thyroid axis in the foetus. This is a transient condition that usually improves in 3–19 months.31,33
- -
Primary hypothyroidism or transient hyperthyrotropinaemia
- -
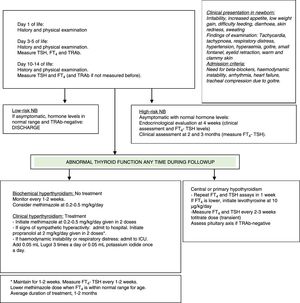
At birth, there may be overt manifestations of hyperthyroidism, but in most cases the onset is delayed (Fig. 1).
- -
Laboratory tests: the presence of TRAb in cord blood is associated with a high risk of developing neonatal hyperthyroidism in the first 2 weeks of life.34 However, the levels of TSH and FT4 in cord blood have no predictive value when it comes to neonatal hyperthyroidism, as they simply reflect the thyroid function in the foetus.
- •
Low-risk newborns: they usually require no followup after 2 weeks post birth (once it is verified that they are healthy and have a normal thyroid function).29
- •
High-risk newborns: in newborns whose mothers did not receive antithyroid medication, the diagnosis is usually made between days 1 and 3 post birth, compared to between days 5 and 15 post birth in newborns whose mothers received antithyroid drugs (the duration of action of MMI is 3 days compared to 24h for PTU).2,29,32 Of all newborns that develop neonatal hyperthyroidism, 95% have onset in the first month of life (most frequently in the first 2 weeks).
- •
Approach to the management of an infant born to a mother with GD or hyperthyroidism (current or past). Pregnant mother: measure TRAb levels in the second to third trimester of pregnancy. Inform the neonatologist of results. If TRAb status positive or unknown: high-risk NB. If TRAb status negative: low-risk NB. Newborn (NB): measure TRAb in cord blood (hormone levels not useful) and monitor.
In newborns, FT4 and TSH levels should be measured between days 3 and 5 post birth (or before if patient is symptomatic), and again between days 10 and 14 post birth (use age-specific reference ranges). If there are no abnormalities in the first 2 weeks of life, performance of clinical assessments at 4 weeks, 2 months and 3 months post birth (with the option of additional biochemical assessment) suffice for the detection of potential late-onset cases.29
- -
Indications for initiating treatment in newborns:
- •
Subclinical biochemical hyperthyroidism: a significant proportion of at-risk newborns are asymptomatic. There is no consensus as to whether they should or not be treated, but it is recommended that they be closely monitored. Some authors propose initiating treatment if FT4 levels exceed 35pmol/L between days 2 and 14 post birth to prevent neonatal hyperthyroidism. Others consider that asymptomatic patients with FT4 levels between 43 and 154pmol/L should not be treated (limiting treatment to patients with symptoms or goitre).2,29,34
- •
Biochemical hyperthyroidism with clinically significant symptoms: there is widespread agreement that treatment should be initiated as soon as the patient develops symptoms to prevent heart failure and long-term sequelae such as intellectual disability.2,29,35
- •
Treatment of neonatal hyperthyroidism:
- -
Both MMI and PTU inhibit thyroid peroxidase and therefore synthesis of thyroid hormones. In addition, PTU inhibits peripheral deiodination of T4 to T3, but due to the risk of liver failure, it should only be offered as a short course in the case of thyroid storm, intolerance to MMI treatment or severe complications such as agranulocytosis.2,33,38 The clinical and biochemical effects of antithyroid medication may not be noticeable for a few days, until thyroid hormone stores are depleted. Initiate MMI when symptoms develop in the context of biochemical hyperthyroidism, generally at 0.2–0.5mg/kg/day divided into two or three doses (a maximum dose of 1mg/kg/day).29
- -
Perform biochemical assessments weekly until the patient stabilises, and every 1–2 weeks thereafter.
- -
If the patient exhibits sympathetic hyperactivity (tachycardia, hypertension, congestive heart failure or poor feeding), add propranolol at 2mg/kg/day administered in two doses for 1 or 2 weeks. These infants need to be admitted to hospital for monitoring.29
- -
In severe or refractory cases, add potassium iodide or Lugol solution. The first dose must be administered at least 1h after the first dose of MMI to prevent iodide from being used in the synthesis of new thyroid hormones (Lugol solution [0.05mL], one drop every 8h, or potassium iodide solution, 1 drop/day).
- -
In the case of haemodynamic instability, respiratory distress or heart failure, add a short course of oral glucocorticoids, which inhibit thyroid hormone secretion and peripheral deiodination of T4 to T3 (prednisolone at 2mg/kg/day administered in 2 doses).29
Side effects of MMI occur in 28% of children, and most are mild, such as mild elevation of liver enzymes, transient leukopenia, gastrointestinal symptoms, skin rashes, arthralgia or myalgia. Severe problems may develop in 0.5% of patients, such as agranulocytosis, Stevens-Johnson syndrome, liver damage or vasculitis.38
- -
Duration of treatment in neonatal hyperthyroidism:
Neonatal hyperthyroidism is self-limiting, as it only lasts for as long as it takes the body to clear the circulating antibodies, whose half-life is 12 days. Hormone levels have normalised by 6 months in most patients, although there are cases that persist up to the age of 12 months. The usual duration of the treatment is 1–2 months. The dose must be tapered off based on the FT4 levels. The decision to end treatment must take into account the clinical condition of the patient and the FT4 levels.29
Clinical management of infants with neonatal hypothyroidismThese cases are usually diagnosed between 4 and 30 days post birth.31,33 Treatment with levothyroxine should be initiated at a dose of 10mcg/kg/day, with testing every 2–4 weeks to taper the dose.
Most patients improve gradually between ages 3 and 19 months, although many authors prefer waiting until the age of 3 years to attempt discontinuation of treatment.2,29
Final recommendations are given in Table 1.
Recommendations.
| Since a high percentage of pregnant women have autoimmune thyroid disease, we recommend getting information from the mother or requesting previous records on the disease |
| TPOAb and TgAb do not seem to block the child's thyroid gland. Newborns of mothers with thyroid autoantibodies and hypothyroidism treated with levothyroxine do not require a thyroid evaluation other than neonatal screening |
| In newborns of mothers with HT, the neonatal screen for congenital hypothyroidism at 48h of life is a non-invasive test that suffices to ensure normal thyroid function in the newborn. Consequently, the presence of hypothyroidism in the pregnant mother does not require a different approach to assess thyroid function in the newborn |
| Children of mothers with GD, past or present, are at risk of developing foetal or neonatal hyperthyroidism, which may have severe consequences on future health. They may also develop transient hypothyroidism or congenital defects secondary to exposure to maternal medication. These cases require careful followup during pregnancy and in the first months of life. Measurement of TRAb levels during pregnancy or in cord blood is very useful to discriminate between newborns at high risk and at low risk of hyperthyroidism |
| Followup is indicated in all children born to mothers with GD, past or current, treated or untreated, especially if antibodies persist |
| Initiation and maintenance of breastfeeding must be promoted in all newborns of mothers with autoimmune thyroid disorders, even if the mother is currently being treated with MMI, PTU or levothyroxine. The sole contraindication is maternal treatment with radioactive iodine during pregnancy |
The authors have no conflicts of interest to declare.
Please cite this article as: Ares Segura S, Temboury Molina C, Chueca Guindulain MJ, Grau Bolado G, Alija Merillas MJ, Caimari Jaume M, et al. Recomendaciones para el diagnóstico y seguimiento del feto y del recién nacido hijo de madre con patología tiroidea autoinmune. Ann Pediatr (Barc). 2018;89:255.