Rapid genome sequencing has been found to be an effective tool for the diagnosis of genetic disorders in neonatal and pediatric intensive care settings, allowing rapid and accurate decision-making and access to personalized care and therapies. Most genetic disorders exhibit significant clinical and genetic heterogeneity, which complicates recognition of the disease and diagnosis by conventional methods. Rapid genome sequencing offers a superior diagnostic yield, improving patient management and reducing diagnostic delay and inpatient care costs. However, there are barriers to its implementation in everyday clinical practice, such as a lack of technological infrastructure and qualified professionals. This study, conducted in a Spanish center, demonstrated the viability of genomic medicine in real-world practice, with a diagnostic yield of 42% and a significant impact on the management of patients in 32.5% of cases.
La secuenciación genómica rápida en unidades de cuidados intensivos neonatales y pediátricos ha demostrado ser una herramienta eficaz para el diagnóstico de enfermedades genéticas, permitiendo la toma de decisiones clínicas rápidas y precisas y el acceso a tratamientos y terapias de precisión. La mayor parte de los trastornos genéticos presentan una gran heterogeneidad clínica y genética que dificulta el reconocimiento de la enfermedad y su diagnóstico con los abordajes convencionales. La secuenciación genómica rápida ofrece un rendimiento diagnostico superior, mejorando el manejo clínico de los pacientes y disminuyendo el tiempo de diagnóstico y el coste hospitalario. Sin embargo, su implementación en la rutina asistencial encuentra barreras como la falta de infraestructura tecnológica y de profesionales especializados. Este estudio realizado en un centro español muestra la viabilidad de la medicina genómica en la práctica clínica, con un rendimiento diagnóstico del 42% y un impacto significativo en el manejo clínico de los pacientes en un 32.5% de los casos.
The number of diagnosable genetic diseases has increased considerably in recent decades and, with it, the demand for faster and more accurate diagnostics. According to the Online Mendelian Inheritance in Man (OMIM) database, there are more than 7400 known Mendelian diseases, covering a broad spectrum of symptoms and affecting multiple body systems. Genetic disorders account for a high proportion of admissions to high-level and complex pediatric care units, such as neonatal intensive care units (NICUs), pediatric intensive care units (PICUs), and pediatric complex chronic disease units. It is estimated that genetic diseases account for approximately 15% of NICU and PICU admissions.1–3 In a study conducted at a pediatric hospital in San Diego (California, United States) with the aim of determining the association of genetic diseases with infant mortality, Owen et al. (2023) found that, among 112 infant deaths recorded between 2015 and 2020, single-locus genetic diseases were the most common identifiable cause of mortality (41%). In addition, effective therapies that could improve outcomes and reduce mortality were available for 31% of these diseases.4 The medical complexity of children admitted to NICUs and PICUs and the high morbidity and mortality in these patients calls for specialized care and rapid clinical responses to deliver effective care, so early diagnosis can be instrumental in establishing a personalized care plan and clinical decision-making in the short and long term.
The recognition of a genetic disease in NICU and PICU patients with a phenotype-based approach, that is, based solely on the clinical presentation, can be challenging. On the one hand, there is considerable genetic and clinical heterogeneity which, together with the low frequency of many genetic disorders and the substantial variation in their phenotypic expression, even among individuals with the same disease, can make it difficult to recognize the disease based on its clinical features. Patients often present with nonspecific symptoms, such as respiratory distress, feeding difficulties or seizures, which can be caused by a broad range of genetic and nongenetic disorders. In addition, certain neurodevelopmental characteristics or facial features, which can be useful in guiding the diagnosis of some genetic diseases, may not be apparent due to the young age of the patients and/or their clinical condition. The physical examination may be impeded by the presence of medical devices and the use of medication, which in turn makes it difficult to characterize the phenotype. Lastly, some children may have atypical presentations or comorbidities, as may be the case with premature infants, further confusing the diagnostic process.
On the other hand, conventional strategies traditionally used for the diagnosis of genetic diseases in neonatal and pediatric intensive care units, based on phenotypes and symptoms, involve the sequential use of different diagnostic techniques, including various laboratory and imaging tests and genetic studies such as microarrays or gene sequencing using Sanger sequencing techniques or targeted gene panels based on massive parallel sequencing techniques. This is a slow and costly approach of limited utility, which in many cases results in the lack of an etiological diagnosis during the patient's stay, constituting a missed opportunity for precision medical and surgical care. In many cases, the patient even dies before the genetic diagnosis can be established.5–9
While it may take weeks to reach a diagnosis with conventional approaches, recent studies have shown that rapid exome sequencing (rES) or rapid genome sequencing (rGS), with a turnaround time of less than 2 weeks, are superior to conventional genetic testing in providing an accurate diagnosis, allowing rapid and informed clinical decision-making. Recently, Kingsmore et al. (2024) reviewed 44 independent studies on the use of rES or rGS in children admitted to the NICU or PICU with diseases of unknown and probable genetic etiology, finding a weighted diagnostic rate of 37% (range, 19%–83%). The proportion of children in whom study results led to consequent changes in management or treatment was 26% (range, 7%–63%).10 There is also evidence that rES and rGS are cost-effective, chiefly because they significantly shorten the length of stay, prevent the performance of costly additional tests and unnecessary invasive procedures and allow optimization of health care resources. The authors estimated a reduction in neat health care costs of $14 265 per child tested by rES or rGS.10
Despite their benefits, there are barriers that hinder the implementation of rES and rGS in health care systems. On one hand, although the cost of sequencing techniques has decreased significantly in the past decade, implementation requires a sizable investment in sequencing equipment and other technological infrastructure that many health care systems may struggle to afford, in addition to specialized personnel. Furthermore, many clinicians are not familiarized with advanced genetic tests and do not consider them a viable alternative in critical situations.
Although the diagnostic yield and impact on clinical management of the implementation of rapid genomic tests in the PICU and NICU settings has been investigated before, few data are available from studies conducted in the Spanish National Health System (NHS), as the limited resources available in public health care systems and the lack of professionals with adequate training and qualifications in genetics hinder the generalized and coordinated application of advanced sequencing techniques in everyday clinical practice.
Application of rapid sequencing techniques in our health care system. Background and current situationTo our knowledge, the only previous study in Spain was conducted by De Castro et al. (2020) in three public tertiary care hospitals of the NHS. It was a prospective study that analyzed the diagnostic yield and turnaround time for a next-generation sequencing panel that included 1870 genes associated with congenital anomalies and early-onset neurologic and metabolic disorders used in 33 neonates with suspected genetic disease. The study found a diagnostic yield of 39.4%, with a median turnaround time of 7.5 days, and testing results led to changes in management in 76.9% of the cases in which a diagnosis was made.11
Material and methodsWe conducted a prospective study in our hospital between January 2020 and June 2023, using trio-rES as the first-line test in pediatric patients admitted to the NICU/PICU to evaluate the feasibility, diagnostic yield and clinical utility of rES. The study was approved by the Research Ethics Committee of the hospital. Patients were selected during their say in the NICU or PICU according to the following inclusion criteria:
- •
Pediatric patients (0–14 years) with clinical manifestations suggestive of a genetic disorder of unknown etiology (eg, multiple congenital anomalies, seizures, hypotonia) or suspicion of a genetic disease with substantial genetic heterogeneity.
- •
Patients with clinical manifestations or a family history suggestive of a genetic etiology, but whose phenotype did not correspond to a specific disease for which a targeted genetic test was available.
- •
Patients who had a defined genetic disorder, but whose clinical manifestations did not allow for a clinical diagnosis.
- •
Children in whom a genetic diagnosis could significantly change clinical management or cases in which genetic counseling could be relevant for the family.
- •
Possibility of obtaining trio DNA samples (patient, mother and father).
Sequencing, bioinformatic processing and data analysis and interpretation were performed at our center, using the in-house bioinformatic workflow and sequencing equipment.
The results were reported to the clinical geneticists that managed the patient. The clinical genetics care team communicated the results to NICU and PICU staff and to families and developed the care plan. To assess clinical utility, we used questionnaires that were completed by the clinical geneticists and/or the referring intensive care doctors. Pretest counseling was delivered in every case, obtaining informed consent, and subsequently proceeding to the collection of peripheral blood samples for trio testing. For the demographic analysis, we collected data on sex, ethnicity, parental history of consanguinity, date of birth, date of admission to the hospital, date of inclusion in the study, unit that referred the patient (NICU/PICU), survival/death and date of death. We collected clinical data using Human Phenotype Ontology (HPO) vocabulary.
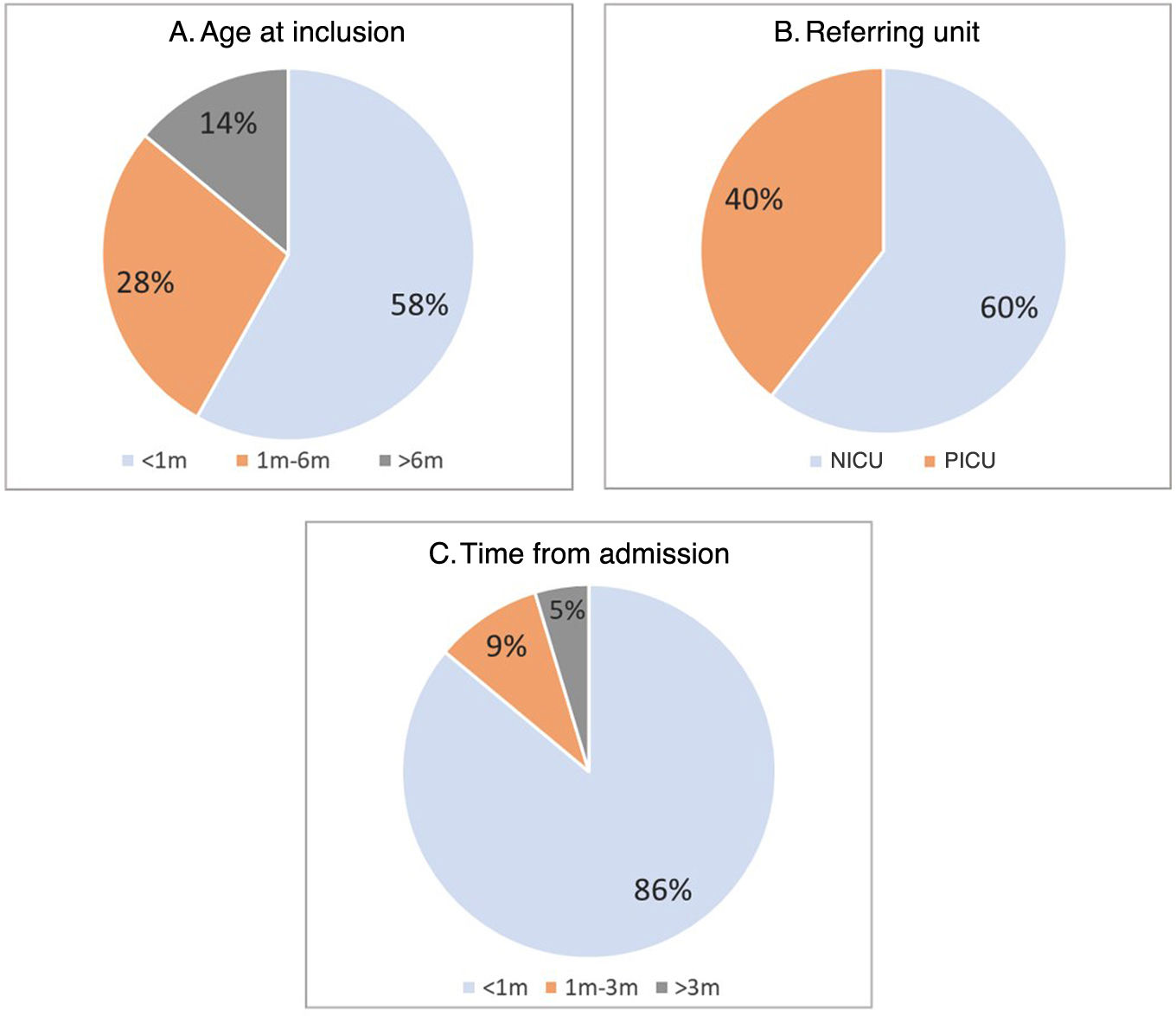
ResultsA total of 43 children (23 male and 20 female) were included. Sixty percent (n = 26) were referred for testing from the NICU and the remaining 40% (n = 17) from the PICU. Fifty-eight percent (n = 25) were recruited in their first month of life (median age, 22 days; range 2 days-4.5 years). Nearly all patients (95.5%; n = 41) enrolled in the study during their first hospital stay, with the exception of two patients who had been hospitalized before, prior to the start of the project. The time elapsed from admission to the hospital to inclusion in the study was less than one month in 86% of cases (n = 37), with a median of 7 days (Fig. 1).
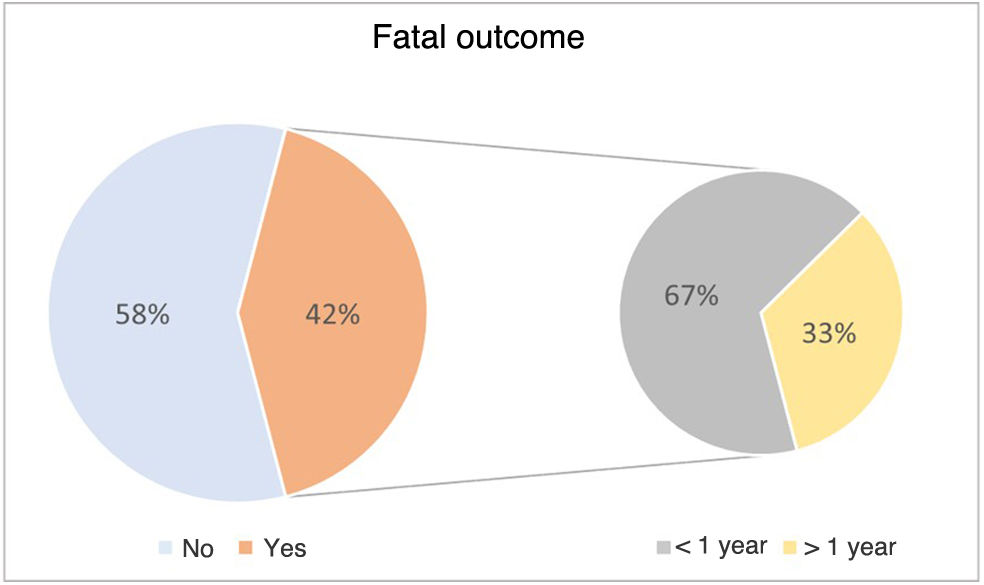
Of the 43 children included in the study, 42% died from the disease (n = 18/43). Furthermore, 67% of the deaths (n = 12/18) occurred before age 1 year (Fig. 2).
The mean time elapsed from submission of trio blood samples to delivery of the reports was 31.3 days, with a standard deviation of 11.6 and a median of 29.5 days.
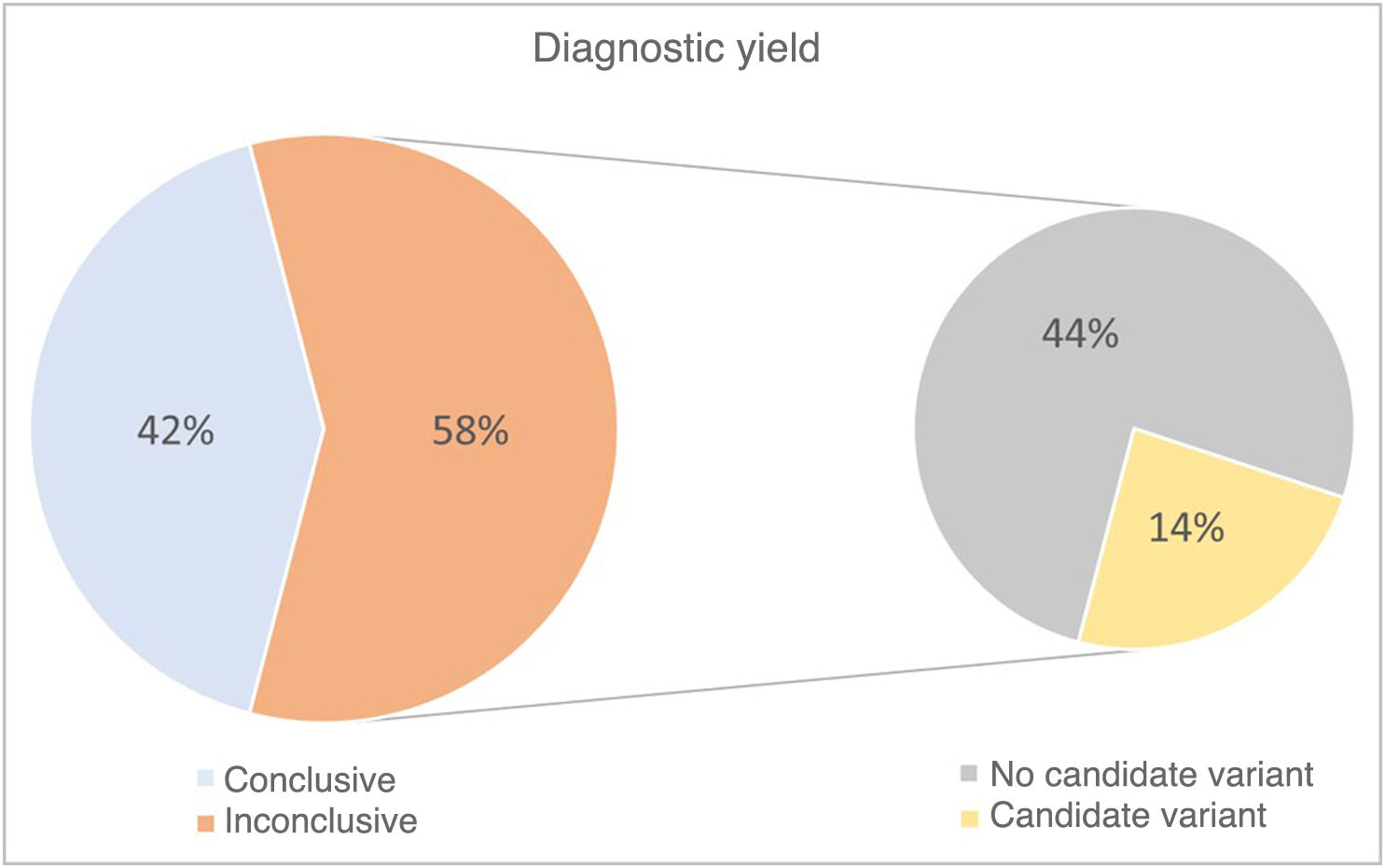
The diagnostic yield was 42%, with the genetic cause identified in 18 of the 43 cases included in the study. A pathogenic variant was identified in the DNA sequence in 16 of the 18 patients (89%), and chromosome abnormalities in the remaining 2 (11%). In addition, in the group of cases in which a diagnosis was not established, variants of uncertain significance were identified in six patients (14%) that will need to be reevaluated as more information becomes available for their interpretation (Fig. 3).
The results of rES led to changes in the management of the patient in 32.5% of cases (n = 14). They allowed rapid adjustment of medication or nutrition in four patients (9%), guided decisions related to the performance of invasive procedures in two cases (5% of total sample) and resulted in referral for follow-up by other medical specialists in eight of the diagnosed cases (19% of total sample).
Testing allowed identification of a higher risk of recurrence in future offspring in all cases diagnosed with sequence variants (n = 16; 37%), including 2 families with autosomal recessive disorders, 13 with autosomal dominant disorders, and one with an X-linked disorder. An early diagnosis was achieved in 50% of patients who died during the study follow-up (n = 8/16), which made it possible to offer genetic counseling to their relatives.
DiscussionSince its initial introduction in 2012, rES and rGS have proven to be useful tools in the management of patients admitted to pediatric or neonatal intensive care units, allowing consistent turnaround times of 3–10 days after sample receipt.10 Several studies have demonstrated their considerable clinical utility and diagnostic yields in patients with suspected genetic disorders, underscoring the impact of early diagnosis on their management. Clinical geneticists play a key role in this process, not only in the evaluation and selection of patients eligible for genomic studies, but also in the clinical interpretation of results, their validation as relevant findings and the integration of molecular diagnosis in the context of the patient’s clinical condition. In spite of the existing evidence, certain factors pose a barrier to the use of these techniques in everyday clinical practice in Spain, such as the associated costs, the lack of qualified professionals for their implementation and the limited familiarity with genetic testing and its perceived usefulness among some of the health care professionals that work in intensive care units.
The findings of the study carried out in our hospital suggest that it is feasible to implement this type of testing in clinical practice with the current resources of the public health care system. The diagnostic yield of 42% found in our cohort is comparable to that of other similar studies, with an average reported diagnostic yield of 37%.10 A mean time to delivery of the final report of 31 days was achieved in our study, and sequencing results led to changes in the management of 32.5% of the total patients in the cohort. These findings suggest that, even with a longer turnaround time compared to other studies, the results of rES can have an impact on patient care. In another study conducted in our health care system in a cohort of similar characteristics, De Castro et al. (2020) reported a mean turnaround time of 7.5 days, and test results led to changes in management in 79.9% of the children in whom a diagnosis was made, which indicates that this percentage was calculated over the subset of patients with a final diagnosis, as opposed to the total cohort.11 Although in many instances changes in management results from establishing a diagnosis, ruling out a genetic etiology can also lead to changes in management. Dimmock et al. (2021) report that in one case in which a genetic cause was not identified, the negative result allowed the withdrawal of life-sustaining treatment in a child who had intractable seizures that required significant respiratory support.12 The nondiagnostic genome made it possible to rule out all treatable genetic causes of neonatal seizures with a high degree of certainty, allowing parents to make the informed decision to transfer their child to palliative care. Thus, taking into account the total cases in the cohort described by De Castro et al. (2020) to calculate the proportion of children in whom testing led to changes in management, the resulting percentage would be 30.3%, a figure that is comparable to the 32.5% identified in our cohort. Another factor worth mentioning that contributed to the differences in turnaround times between the two studies is the equipment used for sequencing. In our hospital, it was the HiSeq 4000 (Illumina), a high-throughput system that can simultaneously sequence 48 exomes per flow cell with a high yield. The system used in the De Castro et al. study (2020) was the NextSeq 500 (Illumina), of lower throughput and with a higher cost of sequencing per sample, but less cumbersome and therefore allowing quicker turnaround times. In our hospital, during the period under study, many genetic diseases were investigated using gene panels. The higher throughput of the sequencing system that was used had an impact on the processing time for exomes, for, in order to optimize run costs, the system was used at full capacity with addition of numerous panel samples per run, resulting in a longer average turnaround time. Our data reflect the difficulty of integrating rES/rGS into routine diagnostic practice, as well as the room for improvement that would make shorter turnaround times possible.
Our findings also demonstrate that early diagnosis facilitates early genetic counseling in most diagnosed cases, enabling family planning, which is crucial for families with a seriously ill or deceased child.
Finally, in addition to sequence variants, we were able to identify chromosome abnormalities and unbalanced structural variants that are usually diagnosed with other approaches, which supports the usefulness of next-generation sequencing techniques as a first-tier diagnostic tool in this group of patients.13
ConclusionsIn children admitted to neonatal or pediatric intensive care units, genomic medicine has the potential to enable faster and more accurate diagnoses, improving clinical management and access to personalized care while facilitating the integration of precision medicine in these care settings, which in turn would result in improved outcomes and a reduction in morbidity and mortality. At the same time, the adoption of genomic medicine in clinical practice poses challenges such as the cost of genetic studies and equipment, the need for infrastructure for data storage, processing, analysis and interpretation, and the protection of privacy and data security, among others. Specialized staff, including bioinformaticians, genetic counselors and clinical and laboratory geneticists, are necessary to ensure that genomic information is managed effectively and ethically in patient care. In particular, the role of the clinical geneticist is key in the interpretation and validation of results from a medical perspective, acting as a bridge between genomic data and their application to patient care, and ensuring that health care decisions derived from genetic studies are founded on the interpretation of a specialist.
It is important for hospitals lacking the necessary infrastructure to implement these genomic techniques in-house to be able to submit samples to reference laboratories in order to guarantee access to these diagnostic procedures. Last of all, studies like the one presented here can build the foundation for a discussion with the competent authorities regarding the inclusion of these studies in the services portfolio of the Spanish public health system.
FundingThis study could be conducted thanks to the funding received from the Instituto de Salud Carlos III through the Health R&D&I projects, AES PI 19/01681, AES PI 22/701823 and AES PI 22/01743, and the European Regional Development Fund.
The authors have no conflicts of interest to declare.