Patent ductus arteriosus (PDA) is a condition that can give rise to an imbalance in the pulmonary to systemic flow ratio (Qp/Qs), with an increase in pulmonary blood flow and a decrease in systemic blood flow. In preterm newborns, it can be associated with important complications such as pulmonary haemorrhage, bronchopulmonary dysplasia, necrotising enterocolitis, impaired renal function and intraventricular haemorrhage, among others.1 Its management and the indications for treatment remain controversial.2 Thanks to the improvement of techniques and the availability of novel devices, percutaneous closure (PC) has become one of the treatment options of choice in cases in which pharmacological closure is unsuccessful.3
In this article, we analyse the experience of our hospital with both approaches over a 10-year period (2012–2022), describing the outcomes of PC and surgical closure (SC). To this end, we included 58 preterm infants with a diagnosis of PDA without associated cardiac defects. We reviewed the health records to collect data on clinical outcomes during the hospital stay, such as the need for respiratory support or inotropic agents and postoperative complications.
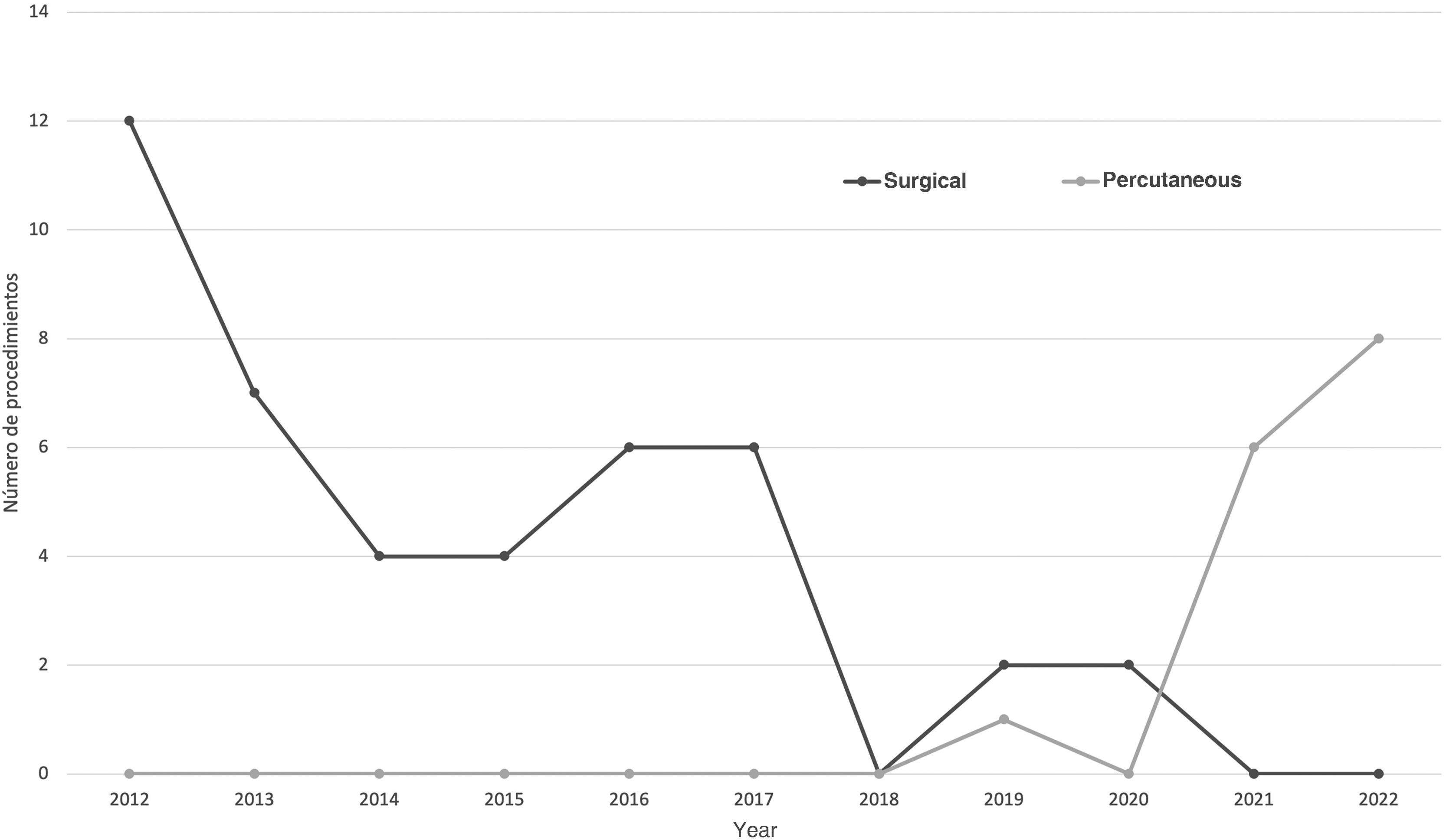
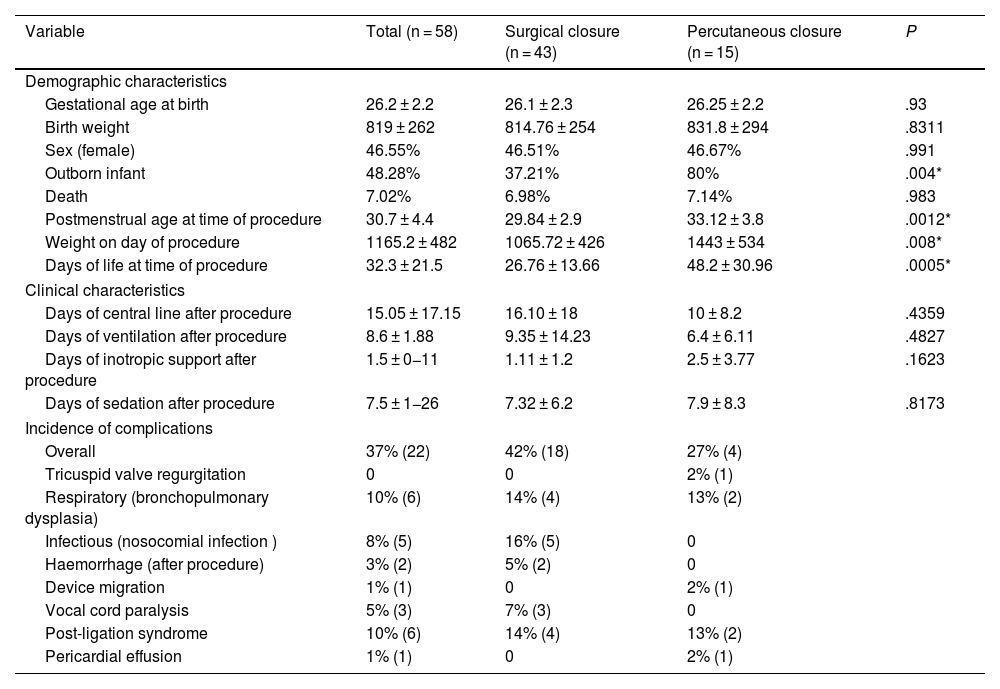
A total of 43 SCs and 15 PCs were performed during the study period. Until 2018, SC was the only procedure performed. In 2019, the first PC took place (Fig. 1). Table 1 presents the characteristics and main clinical outcomes analysed in the sample.
Characteristics of the sample. Complications by group.
| Variable | Total (n = 58) | Surgical closure (n = 43) | Percutaneous closure (n = 15) | P |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Gestational age at birth | 26.2 ± 2.2 | 26.1 ± 2.3 | 26.25 ± 2.2 | .93 |
| Birth weight | 819 ± 262 | 814.76 ± 254 | 831.8 ± 294 | .8311 |
| Sex (female) | 46.55% | 46.51% | 46.67% | .991 |
| Outborn infant | 48.28% | 37.21% | 80% | .004* |
| Death | 7.02% | 6.98% | 7.14% | .983 |
| Postmenstrual age at time of procedure | 30.7 ± 4.4 | 29.84 ± 2.9 | 33.12 ± 3.8 | .0012* |
| Weight on day of procedure | 1165.2 ± 482 | 1065.72 ± 426 | 1443 ± 534 | .008* |
| Days of life at time of procedure | 32.3 ± 21.5 | 26.76 ± 13.66 | 48.2 ± 30.96 | .0005* |
| Clinical characteristics | ||||
| Days of central line after procedure | 15.05 ± 17.15 | 16.10 ± 18 | 10 ± 8.2 | .4359 |
| Days of ventilation after procedure | 8.6 ± 1.88 | 9.35 ± 14.23 | 6.4 ± 6.11 | .4827 |
| Days of inotropic support after procedure | 1.5 ± 0−11 | 1.11 ± 1.2 | 2.5 ± 3.77 | .1623 |
| Days of sedation after procedure | 7.5 ± 1−26 | 7.32 ± 6.2 | 7.9 ± 8.3 | .8173 |
| Incidence of complications | ||||
| Overall | 37% (22) | 42% (18) | 27% (4) | |
| Tricuspid valve regurgitation | 0 | 0 | 2% (1) | |
| Respiratory (bronchopulmonary dysplasia) | 10% (6) | 14% (4) | 13% (2) | |
| Infectious (nosocomial infection ) | 8% (5) | 16% (5) | 0 | |
| Haemorrhage (after procedure) | 3% (2) | 5% (2) | 0 | |
| Device migration | 1% (1) | 0 | 2% (1) | |
| Vocal cord paralysis | 5% (3) | 7% (3) | 0 | |
| Post-ligation syndrome | 10% (6) | 14% (4) | 13% (2) | |
| Pericardial effusion | 1% (1) | 0 | 2% (1) | |
Values expressed as percentage or mean ± standard deviation. (*) Statistically significant result.
We did not compare both techniques since they involved 2 historical cohorts that were very different. We found higher values for patients in the PC group for postmenstrual age (P = .0012), weight (P = .008) and days of chronological age (P = .0005) at the time of the procedure. In spite of this, the clinical outcomes in each group based on some of the main indicators of severity (central line days, mechanical ventilation days, days of inotropic treatment, etc) did not differ between the two cohorts.
The overall incidence of complications in our study, including both techniques, was 38%. Table 1 describes the most frequent complications.
Some complications are specific to one of the procedures. In the case of SC, the most frequently described complications are post-ligation syndrome, pneumothorax, chylothorax, atelectasis, infection, bleeding, diaphragmatic paralysis and vocal cord paralysis secondary to damage of the left recurrent laryngeal nerve.4 In our sample, the incidence of complications in this group was 42%, and the most frequent complications were post-ligation syndrome, infections and respiratory complications (bronchopulmonary dysplasia), with an overall percentage (44%) similar to previous reports in the literature.4 We ought to highlight that there were no cases of chylothorax, pneumothorax or surgical wound infection.
As regards the complications associated with PC, device embolization, obstruction of the aorta or left pulmonary artery by a protruding device, tricuspid valve damage and persistent residual ductal flow are the most common ones. The literature describes a rate of complications of up to 33%, which is slightly higher than the one observed in our cohort, of 27%.5
Lastly, the mortality in the sample was 7%, which was consistent with the previous literature.6
In this article, we describe the experience in our hospital with nonpharmacological PDA closure through these 2 therapeutic approaches over a period of 10 years. During this time, we observed a change in the management of cases refractory to medical treatment, switching from SC to PC (Fig. 1). These trend has also been described by other authors and it is associated with the development and improvement of PC.4 We also ought to mention that postmenstrual age, the days of chronological age and the weight of patients at the time of the procedure were significantly greater in the PC group. This could be explained by the more restrictive indications for PDA closure currently applied in most neonatal units.
Percutaneous closure of PDA is the procedure of choice for definitive ductal closure in adults, children and infants weighing more than 6 kg. In the past decade, the evidence on the safety and viability of percutaneous PDA closure in early childhood and even in preterm infants has been mounting. Given the favourable outcomes and low rate of complications, at present the minimum weight considered required in infants for performing the procedure continues to decrease. Several clinical trials have contributed safety and efficacy data for PC devices in patients weighing between 700 g and 2 kg, and PC has become the standard of care in the management of PDA for this subset of patients in many centres.3
The introduction of PC as a treatment for PDA in our hospital appears to be an effective strategy with a rate of complications similar to that reported in the recent literature.5
The long-term follow-up of these patients will be of vital importance for the purpose of achieving more complete understanding of the safety and effectiveness of both techniques.
FundingThis research did not receive any external funding.