After reading with interest the excellent article “Thirdhand smoke and other challenges of tobacco control in the paediatric population”,1 I consider important to specifically mention the new heated tobacco products (HTPs) that have been recently introduced in the market.
According to the World Health Organization, HTPs are processed tobacco products that are heated instead of burnt. Upon heating, they produced aerosols containing nicotine and other chemical substances, from additives and flavourings, that are inhaled by the user through the mouth. At present, only one such product is distributed in Spain (IQOS, produced by Philip Morris), which has 3 basic components: a disposable “stick” containing processed tobacco, a battery-power heating holder and a charger. Heated tobacco products must not be confused with e-cigarettes, as the former contain tobacco and the latter are cartridges filled with fluid that may or may not contain nicotine, but does not contain tobacco.
In the European Union, HTPs are considered “novel tobacco products”, as defined in Directive 2014/40/UE of the European Parliament. In Spain, since tobacco sticks are considered a tobacco product, they are subject to Law 28/2005 regulating public health measures against tobacco use.2 Recently, some authors have proposed ending the regulatory privileges that HTPs enjoy.3
The data on the compounds generated in heated tobacco and found in the resulting aerosols are still scarce, but it is that the use of HTPs entails the consumption of various emitted toxic and carcinogenic substances associated with tobacco use, such as nitrosamines and cyclic hydrocarbons as well as toxic gases. According to a report issued by the Food and Drug Administration (FDA) of the United States,4 the existing evidence is insufficient to support that HTPs are less harmful to health than conventional tobacco products for either consumers nor individuals exposed to their emissions, especially children. The report also rejected the classification of HTPs as modified risk tobacco products.4
Several articles by independent authors without affiliations to the tobacco industry have reported that the aerosols produced by IQOS have a similar deleterious impact on vascular endothelial function compared to conventional cigarettes. These authors have reported the presence of pulmonary inflammation, remodelling of the airway epithelium and immunomodulation, while at the hepatic level, they have reported an increase in bilirubin levels.
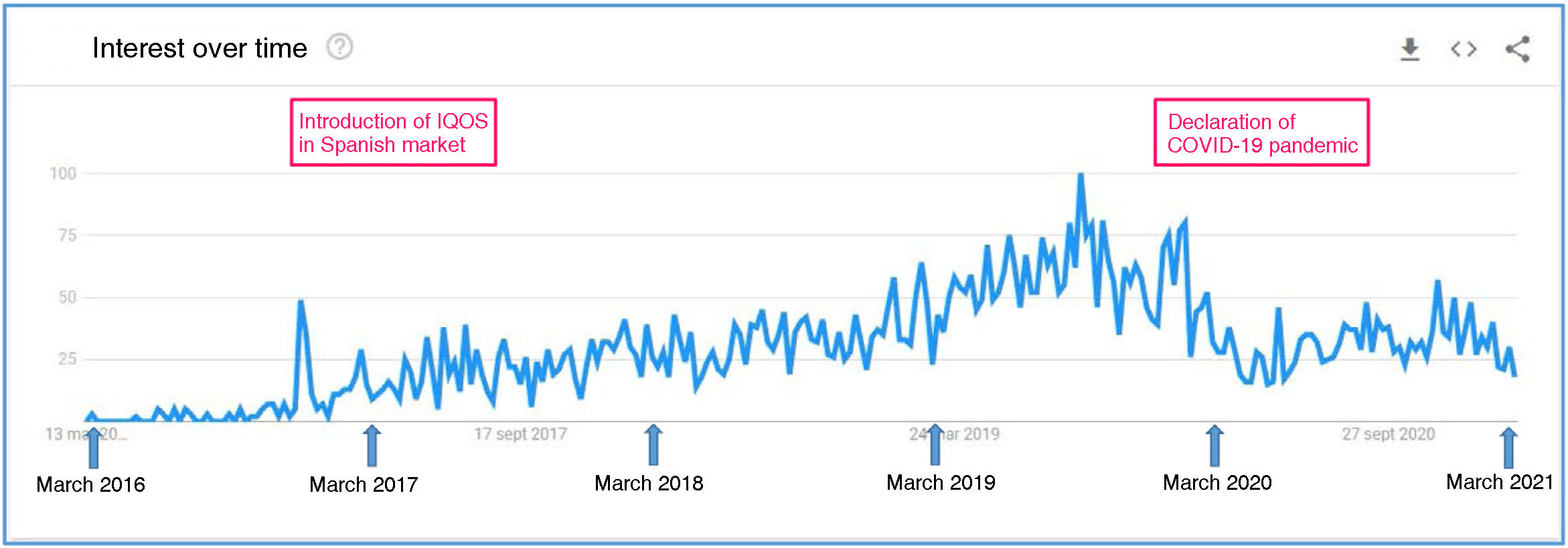
The tobacco industry is deploying an aggressive marketing campaign, advertising IQOS as a product that is less harmful to health, emphasising its cleanliness and with a high-tech appearance. This strategy makes this product very appealing, especially to youth. In fact, the number of online searches for this product has increased significantly in Spain since late 2016 (Fig. 1).
Volume of online searches. Search term: IQOS (March 2016–March 2021). There was a progressive increase in Google searches from late 2016, coinciding with the introduction of IQOS in the Spanish market, with a subsequent decreasing trend from March 2020, coinciding with the declaration of the COVID-19 pandemic.
Source: Google Trends.
In light of the above, it is essential that the population be informed of the risks of consuming these products and to continue implementing strategies to prevent tobacco use.