Neuroendocrine cell hyperplasia of infancy (NEHI) is one of the diseases included in the children interstitial lung disease (ChILD) category. In Spain, the incidence of ChILD is 8.18 new cases per million children per year (. 6,28−10.48) and the prevalence is 46.53 cases per million children (95% CI, 41.81–51.62), and NEHI is the most frequent ChILD in infants aged 1–12 months.1 Since it was first described in 2001,2 more details about its clinical and radiological features and outcomes have come to light, and, while affected children require supplemental oxygen for several years, the long-term prognosis is generally good.3
We present the cases of six patients given a diagnosis of NEHI in our hospital between 2013 and 2024 (Table 1). There was a predominance of the male sex (5:6). None of the patients had a relevant family history. One was born at 36 weeks (late preterm) of gestation age with adequate weight for gestational age (2760 g). All of them had onset between 2 and 5 months post birth (mean age, 3 months) with bilateral crackles on chest auscultation (6/6), tachypnea (6/6), chest retractions (6/6), daytime hypoxemia (2/6), nighttime hypoxemia (5/6), weight faltering (4/6) and pectus excavatum (1/6). Four patients required hospitalization due to respiratory symptoms prior to diagnosis, which prompted the subsequent evaluation. The other two were referred to the outpatient pulmonology clinic for evaluation of persistent tachypnea and weight faltering. Patients were evaluated by means of the NEHI clinical score,4 which assesses the presence or absence (absent = 0 points; present = 1 point) of the following 10 items: tachypnea, failure to thrive, hypoxemia, abnormal chest wall, absence of cough when well, absence of wheeze when well, symptom onset before 12 months, cracklets, retractions and absence of clubbing, with a mean score of 8.16 points (scores ≥7 are strongly suggestive of NEHI). The tests performed included chest X rays (6/6), complete blood count, blood chemistry panel and immune system evaluation (6/6), cardiovascular assessment (6/6), chest computed tomography (CT) (6/6); lung biopsy (2/6), bronchoscopy with bronchoalveolar lavage (2/6), pH test (2/6) and assessment dysphagia by means of a videofluoroscopic swallow study (1/6). All tests were normal in the six patients with the exception of the chest radiograph, which revealed diffuse peribronchial thickening with (5/6) or without bilateral abnormal density (1/6), and the chest CT, with ground glass opacities most prominent in the upper lobes, right middle lobe and lingula (6/6) and inversion of the normal ventilatory ratio (6/6). A lung biopsy was performed via thoracoscopy in the two patients managed earliest (2013 and 2014), both aged 9–10 months at the time, with and increased neuroendocrine cell numbers and large neuroepithelial bodies within bronchiole walls visualized through immunostaining with neuroendocrine markers (synaptophysin and chromogranin). The diagnosis was based on the CT and lung biopsy findings in two patients and on the CT findings in four. The median age at diagnosis was 14 months, with a range of 5–22 months.
Clinical and radiological characteristics and outcomes of the six patients.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Sex | Male | Male | Male | Male | Male | Female |
| Family history | No | No | No | No | No | No |
| Gestational age (Weeks) | 37 | 40 | 38 | 37+6 | 36 | 37+3 |
| Birth weight (g) | 2900 | 3310 | 3220 | 2810 | 2750 | 3010 |
| Age at onset (months) | 3 | 1 | 2 | 5 | 5 | 1 |
| Initial symptoms | ||||||
| Tachypnea | + | + | + | + | + | + |
| Crackles | + | – | + | – | + | + |
| Retractions | + | + | + | + | + | + |
| Hypoxemia | + | + | + | + | + | + |
| weight faltering | – | + | + | + | – | + |
| Chest wall abnormalities | – | – | – | – | – | + |
| NEHI score | 8 | 8 | 9 | 8 | 7 | 9 |
| Chest CT | ||||||
| Age at testing (months) | 9 | 7 | 22 | 18 | 19 | 5 |
| Ground glass opacity | + | + | + | + | + | + |
| inversion of the normal ventilatory ratio | + | + | + | + | + | + |
| Lung biopsy | Yes | Yes | No | No | No | No |
| Age at testing (months) | 10 | 9 | ||||
| Age at diagnosis (months) | 10 | 9 | 22 | 18 | 19 | 5 |
| Year of diagnosis | 2013 | 2014 | 2016 | 2017 | 2018 | 2024 |
| Treatment | ||||||
| Oxygen | + (nighttime) | + (nighttime) | + | + (nighttime) | + (nighttime) | + |
| Rescue salbutamol | + | + | + | + | + | – |
| Inhaled steroids | + | – | + | + | + | – |
| Rescue oral steroids | + | – | + | + | + | – |
| Recurrent wheezing | Yes | No | YES | YES | YES | No |
| Age (months) at withdrawal of oxygen therapy | ||||||
| 24 hours/day | – | – | 33 | – | – | |
| Nighttime | 52 | 12 | 94 | 29 | 84 | Ongoing |
| Occasional | 52 | 12 | 94 | 48 | 84 | |
Abbreviation: NEHI, neuroendocrine cell hyperplasia of infancy.
All patients received supplemental oxygen though nasal prongs from diagnosis, either around the clock (2/6) or only at night (4/6). Other treatments including maintenance inhaled steroid therapy for recurrent wheezing and inhaled salbutamol, alone or combined with oral steroid therapy, for management of acute bronchitis (4/6). In the five patients currently aged more than 6 years, respiratory symptoms resolved at a mean age of 18 months, and oxygen therapy could be permanently withdrawn at a mean age of 52 months, and these patients currently have weights and heights in the normal range for age.
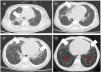
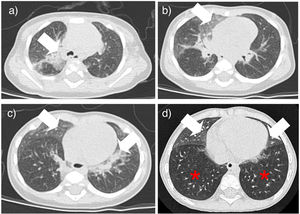
The six cases of NEHI presented here demonstrates that this is one of the children interstitial lung diseases with the most favorable prognosis. The delay in diagnosis may be related to the nonspecificity of the initial respiratory symptoms and the low level of suspicion resulting from the low frequency of the disease,3 and diagnosis is based on the respiratory symptoms assessed by the NEHI clinical score, which manifest between age 1 and 12 months (usually by age 6 months) and the typical chest CT scan features (ground glass opacities predominantly in lingula and/or air trapping with inversion of the normal ventilatory ratio5 (Fig. 1). Although a possible genetic etiology has been hypothesized, a causative gene has yet to be clearly identified, and none of our patients underwent genetic testing. The lung biopsy is reserved for cases with uncharacteristic features.6