Left pulmonary artery sling (LPAS) is a rare anomaly in which the left pulmonary artery (PA), arises from the right PA instead of the main PA, running over the right main bronchus and between the trachea and esophagus to reach the left hilum, forming a vascular ring.1,2 This results in the anatomical obstruction of the right main bronchus, the trachea or both.1 The majority of patients present respiratory symptoms (wheezing, stridor or cough) and recurrent respiratory infections of varying severity, depending on the degree of concomitant tracheal stenosis (TS), which determines its clinical course and prognosis.2 More patients are currently surviving to adulthood owing to improved neonatal care and surgical management, although it may still be misdiagnosed prenatally due to the limited knowledge of this condition.3 Thus, the aim of this study was to describe 12 paediatric patients with LPAS in order to increase awareness of this condition among paediatricians, with the ultimate objective that LPAS be included in the differential diagnosis of with persistent or recurrent respiratory symptoms in infants.
We conducted a retrospective observational study that included 12 paediatric patients with LPAS (October 2003-September 2024). Quantitative data, which did not follow a normal distribution, are expressed as a median and interquartile range (IQR). Categorical data are presented as absolute frequencies (n) and percentages (%).
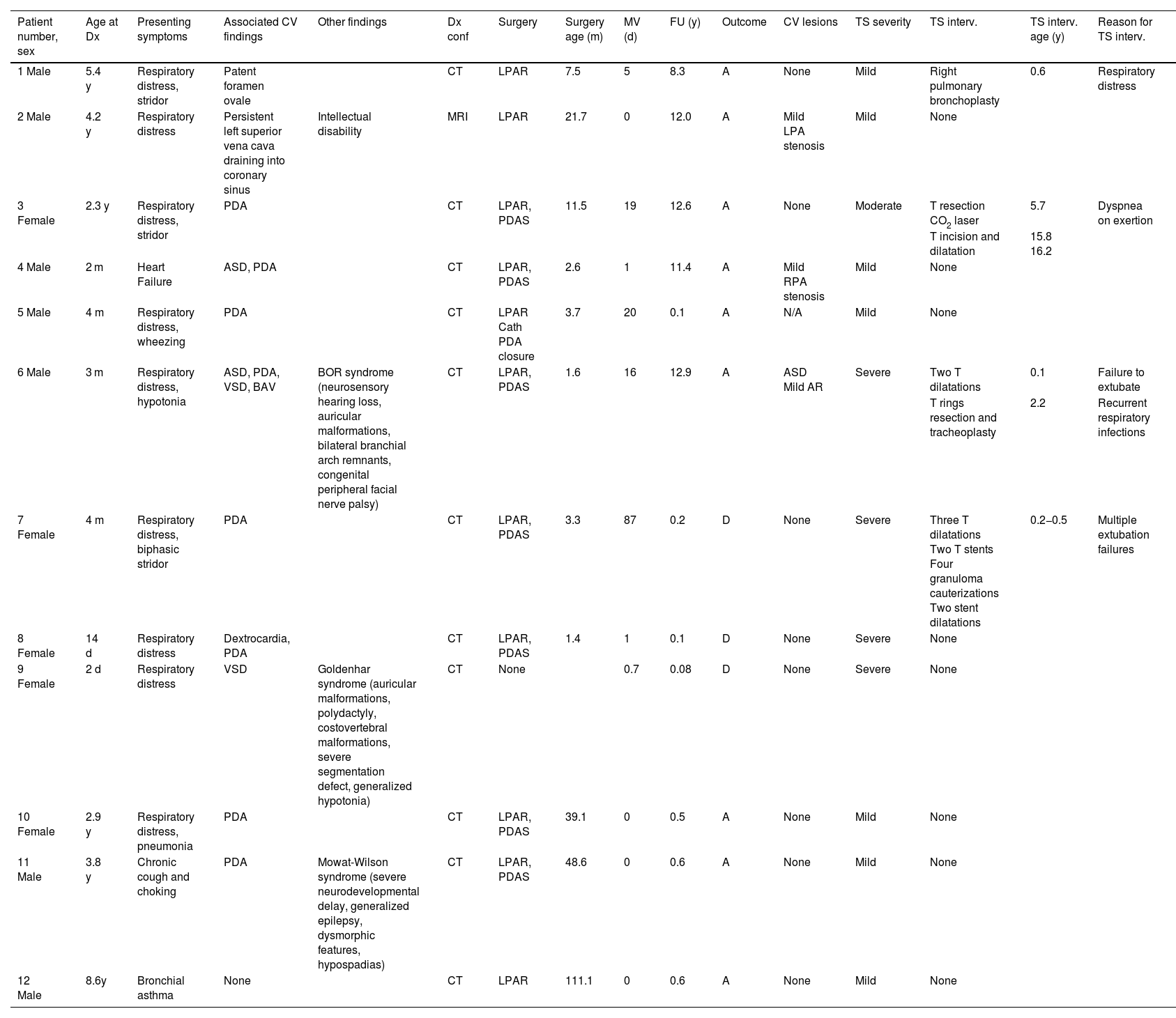
Table 1 presents the demographic and clinical characteristics of the patients. The median age at diagnosis was 4.3 months (IQR, 0.9–43.2 months); the duration of follow-up was 0.6 years (IQR, 0.2–12.0 years). All patients were diagnosed postnatally with one exception (patient 8).
Demographic and clinical characteristics of our cohort of patients with left pulmonary artery sling.
| Patient number, sex | Age at Dx | Presenting symptoms | Associated CV findings | Other findings | Dx conf | Surgery | Surgery age (m) | MV (d) | FU (y) | Outcome | CV lesions | TS severity | TS interv. | TS interv. age (y) | Reason for TS interv. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Male | 5.4 y | Respiratory distress, stridor | Patent foramen ovale | CT | LPAR | 7.5 | 5 | 8.3 | A | None | Mild | Right pulmonary bronchoplasty | 0.6 | Respiratory distress | |
| 2 Male | 4.2 y | Respiratory distress | Persistent left superior vena cava draining into coronary sinus | Intellectual disability | MRI | LPAR | 21.7 | 0 | 12.0 | A | Mild LPA stenosis | Mild | None | ||
| 3 Female | 2.3 y | Respiratory distress, stridor | PDA | CT | LPAR, PDAS | 11.5 | 19 | 12.6 | A | None | Moderate | T resection CO2 laser | 5.7 | Dyspnea on exertion | |
| T incision and dilatation | 15.8 16.2 | ||||||||||||||
| 4 Male | 2 m | Heart Failure | ASD, PDA | CT | LPAR, PDAS | 2.6 | 1 | 11.4 | A | Mild RPA stenosis | Mild | None | |||
| 5 Male | 4 m | Respiratory distress, wheezing | PDA | CT | LPAR Cath PDA closure | 3.7 | 20 | 0.1 | A | N/A | Mild | None | |||
| 6 Male | 3 m | Respiratory distress, hypotonia | ASD, PDA, VSD, BAV | BOR syndrome (neurosensory hearing loss, auricular malformations, bilateral branchial arch remnants, congenital peripheral facial nerve palsy) | CT | LPAR, PDAS | 1.6 | 16 | 12.9 | A | ASD Mild AR | Severe | Two T dilatations | 0.1 | Failure to extubate |
| T rings resection and tracheoplasty | 2.2 | Recurrent respiratory infections | |||||||||||||
| 7 Female | 4 m | Respiratory distress, biphasic stridor | PDA | CT | LPAR, PDAS | 3.3 | 87 | 0.2 | D | None | Severe | Three T dilatations Two T stents Four granuloma cauterizations Two stent dilatations | 0.2−0.5 | Multiple extubation failures | |
| 8 Female | 14 d | Respiratory distress | Dextrocardia, PDA | CT | LPAR, PDAS | 1.4 | 1 | 0.1 | D | None | Severe | None | |||
| 9 Female | 2 d | Respiratory distress | VSD | Goldenhar syndrome (auricular malformations, polydactyly, costovertebral malformations, severe segmentation defect, generalized hypotonia) | CT | None | 0.7 | 0.08 | D | None | Severe | None | |||
| 10 Female | 2.9 y | Respiratory distress, pneumonia | PDA | CT | LPAR, PDAS | 39.1 | 0 | 0.5 | A | None | Mild | None | |||
| 11 Male | 3.8 y | Chronic cough and choking | PDA | Mowat-Wilson syndrome (severe neurodevelopmental delay, generalized epilepsy, dysmorphic features, hypospadias) | CT | LPAR, PDAS | 48.6 | 0 | 0.6 | A | None | Mild | None | ||
| 12 Male | 8.6y | Bronchial asthma | None | CT | LPAR | 111.1 | 0 | 0.6 | A | None | Mild | None |
A, alive; AR, aortic regurgitation; ASD, atrial septal defect; BAV, bicuspid aortic valve; BOR, branchio-oto-renal; Cath, catheterization; Conf, confirmation; CT, computed tomography; CV, cardiovascular; D, deceased; d, days; Dx, diagnosis; FU, follow-up; Interv, intervention; LPAR, reimplantation of the left pulmonary artery; m, months; MRI, magnetic resonance imaging; MV, mechanical ventilation; N, number; N/A, not available; PDA, patent ductus arteriosus; PDAS, patent ductus arteriosus section; T, tracheal; TS, tracheal stenosis; VSD, ventricular septal defect; y, years.
Eleven patients (83.3%) underwent LPAS repair (at a median age of 5.6 [IQR, 1.8–34.7] months). The left PA was transected from its origin on the right PA, translocated anteriorly, with relief of tracheal compression, and directly anastomosed to the main PA.
All patients had TS (Table 1) which did not improve in 3 out of the 11 patients that underwent LPAS repair (patients 3, 6 and 7; Fig. 1). Patient 7 required several surgical interventions for management of tracheal stenosis and granuloma following multiple extubation failures in the postoperative period. After one such episode, she developed respiratory failure due to complete airway obstruction by the granuloma and died 87 days after the surgery (at age 6.2 months). Patient 9 (aged 1 month) could not undergo LPAS repair because severe TS and complete tracheal rings made intubation impossible (Supplementary Figure 2). Since the patient had multiple concomitant malformations and had a fatal prognosis, the decision was made in agreement with the parents to limit life-sustaining treatment.
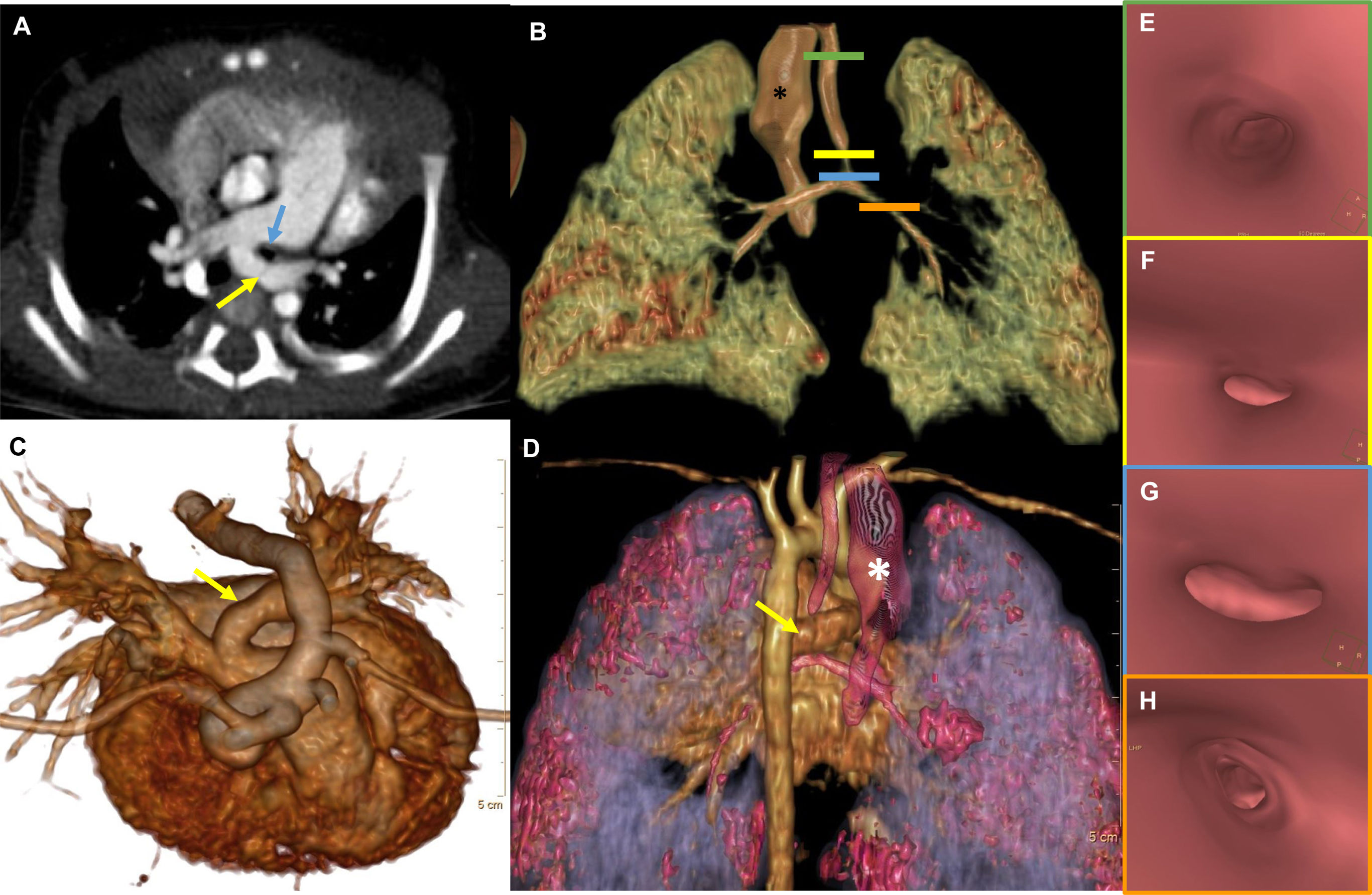
Computed tomography (CT) and volume rendering (VR) images of patient 6 showing left pulmonary artery sling.
Axial CT image (A) showing a left pulmonary artery (yellow arrow) arising from the right pulmonary artery and surrounding the left main bronchus (blue arrow), consistent with left pulmonary artery sling. Volume rendering images (B, C, D) show the course of the left pulmonary artery (yellow arrow; C, D) in relation to the airways (B, C, D). Dilatation of the esophagus (white asterisk) can be perceived (D). Virtual endoscopy images (E, F, G, H) of the trachea (E), showing significant stenosis of the trachea (F), tracheal bifurcation (G) and left bronchus (H).
Three patients (25.0%) died during the postoperative period (patients 7, 8 and 9). Patient 8 died in the context of suspected sepsis on day one post surgery (age, 1.4 months).
The median duration of follow-up was 0.6 years (IQR, 0.2–12.0 years) overall and 10.0 years (IQR, 0.5–12.5 years) in survivors. At the end of the study period, at a median of 4.4 years (IQR, 0.1–13.9 years) post surgery, all operated patients were alive and asymptomatic.
To our knowledge, this is the largest LPAS study conducted in Spain.3 As noted above, the most frequent clinical presentation of LPAS is respiratory symptoms (stridor, dyspnea or wheezing) masquerading as recurrent pneumonia or bronchiolitis.4,5 Thus, the majority of our patients presented with respiratory symptoms.
Tracheal stenosis is present in 60%–100% of patients with LPAS,4,5 which was also the case in our study. Preoperative assessment of TS is critical for optimal surgical planning, and slide tracheoplasty is the most widely used intervention.6 At present, there is a lack of consensus regarding the specific indications for tracheal intervention in LPAS.4,7 Still, Muthialu et al. reported that LPAS repair achieved good outcomes even in the presence of airway malformations.6 None of the patients managed with LPAS repair in our sample underwent tracheoplasty in the same intervention, although three of them required tracheal surgery at a later time. This might be partially explained by the fact that, in our center, the indication for tracheal surgery is based on the difficulties in ventilation and/or impossibility to wean the patient off mechanical ventilation. Furthermore, TS is associated with a 11%–45% mortality rate, in line with our results (16.7%).5 It is possible that a more aggressive approach towards TS, with performance of tracheoplasty at the time of LPAS repair, could have prevented the fatal outcome.
Goldstein et al. previously reported a high incidence of PA stenosis at the reimplantation site of the LPA requiring intervention in 45% of affected patients.8 In contrast to this, the incidence in our study was lower (8.3%).
In conclusion, the diagnosis of LPAS is challenging due to its rarity and heterogeneous manifestations. Nonetheless, pediatricians should consider this entity in the differential diagnosis of infants presenting with respiratory symptoms, as timely diagnosis is paramount for prompt initiation of treatment and follow-up to diminish post-operative residual lesions and morbidity, as our results demonstrate. Besides, TS repair has to be considered in patients with LPAS, as it can result in death. Further studies are required, to improve not only the consensus regarding the surgical management of TS surgical, but also our understanding of the etiology of LPAS and the associated anomalies. These will enhance its clinical characterization, increasing clinicians’ awareness of this condition.
Ethical considerationsThe authors assert that all procedures contributing to this work complied with the ethical standards of the applicable national guidelines and was approved by the ethics committee of the Hospital Sant Joan de Déu.
FundingThis research did not receive any external funding.
The authors have no conflicts of interest to declare.