Thiopurines are drugs widely used in patients for the maintenance of remission in inflammatory bowel disease. The optimal plasma levels are known, but there is controversy about whether the need for other drugs is reduced or cost-effective. The aim of this study is to describe the use of the optimised treatment with thiopurines in paediatric patients with inflammatory bowel disease followed up in this Unit since the introduction of determining the drug levels.
Material and methodsA descriptive retrospective study was conducted in which the plasma values of 6-thioguanine (6-TGN), 6-methyl-mercapto-purine (6-MMP), and their ratios were analysed using liquid chromatography. Other variables were collected, such as clinical status, analytical and demographic variables of patients with inflammatory bowel disease followed up in this Unit.
ResultsA total of 72 patients were included, and 140 determinations of metabolites were performed. The 6-TGN levels were found to below the therapeutic range in 61.5% of patients (in 7 cases due to lack of adherence to therapy), and 6-MMP was in the toxicity range in 7.4%. After the determination of 77 specimens, some action was taken, such as modifying the dose, change of formula, or withdrawing the drug. Only 9 patients were scaled to a biological drug (13.4% of the total on single therapy). No association was found between the activity of the disease and the thiopurine levels.
ConclusionsIn our experience, the monitoring of thiopurine levels helped to modify the drug dose that the patient received, adjusting their therapeutic levels, and potentially avoiding the addition of new drugs.
Las tiopurinas son fármacos muy empleados para el mantenimiento de la remisión en pacientes con enfermedad inflamatoria intestinal. Se conocen cuáles son los niveles plasmáticos óptimos, y existe controversia acerca de si reducen la necesidad de otros fármacos o son coste-efectivos. El objetivo de nuestro estudio fue describir el uso del tratamiento optimizado con tiopurínicos en pacientes pediátricos con enfermedad inflamatoria intestinal seguidos en nuestra unidad desde la implementación de la determinación de niveles de fármaco.
Material y métodosEstudio descriptivo retrospectivo en el que se analizaron valores en plasma mediante cromatografía líquida de 6-tioguanina (6-TGN), 6-metilmercaptopurina (6-MMP) y sus cocientes, así como estado clínico y variables analíticas y demográficas de pacientes con enfermedad inflamatoria intestinal en seguimiento en nuestra unidad.
ResultadosSe incluyeron 72 pacientes y se realizaron 140 determinaciones de metabolitos. En el 61,5% de las determinaciones los niveles de 6-TGN se encontraban por debajo del rango terapéutico (en 7casos debido a falta de adherencia terapéutica), y en el 7,4% de las de 6-MMP estaban en rango de toxicidad. Tras la determinación de 77 muestras se tomó alguna actitud derivada, procediéndose a la modificación de dosis, al cambio de formulación o a la suspensión del fármaco. Únicamente 9pacientes escalaron a fármaco biológico (13,4% del total que estaban en monoterapia). No se encontró relación entre la actividad de la enfermedad y los niveles de tiopurínicos.
ConclusionesEn nuestra experiencia la monitorización de niveles de tiopurinas ayudó a modificar la dosis de fármaco que recibía el paciente, adecuando sus niveles terapéuticos y evitando potencialmente la adición de nuevos fármacos.
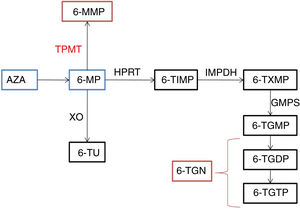
Thiopurines are purine analogues frequently used to maintain remission in inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn disease (CD). Azathioprine (AZA) and 6-mercaptopurin (6-MP) are two of them.1 These 2 agents do not have a direct therapeutic effect and are therefore considered prodrugs. Azathioprine is converted by the enzyme glutathione S-transferase to 6-MP, generating the metabolites 6-thioguanine (6-TGN) and 6-methylmercaptopurine (6-MMP) (Fig. 1). The therapeutic effect of AZA is attributed to 6-TGN, which by inhibiting the enzyme Rac1 induces T cell apoptosis and reduces inflammation.2 In addition, since they are purine analogues, they interfere with the synthesis of proteins and nucleic acids. They are considered immunomodulatory drugs that act intracellularly and have a delayed effect that peaks 10 to 12 weeks from initiation of therapy.3 Their toxicity is mainly attributed to 6-MMP, but 6-TGN may also contribute to it.4 Thiopurine methyltransferase (TPMT) is a key enzyme when it comes to cytotoxicity, as it catalyses the conversion of 6-MP to 6-MMP and the levels of the toxic metabolite will vary depending on the level of activity of this enzyme. Thiopurine methyltransferase variants corresponding to a low enzymatic activity are considered a risk factor for drug toxicity, and therefore thiopurines should not be used in patients homozygous for these variants.3 However, myelotoxicity can occur despite normal TPMT activity5,6 and measurement of TPMT activity can only help establish the increased risk of myelosuppression in patients homozygous for a low-activity variant, who in Spain amount to less than 0.5% of the total population.7
Thiopurine metabolic pathway.
6-MMP, 6-methylmercaptopurine; 6-MP, 6-mercaptopurine; 6-TGDP: 6-thioguanine diphosphate; 6-TGMP: 6-tioguanina monophosphate; 6-TGTP: 6-tioguanina triphosphate; 6-TIMP: 6-metiltioinosinato; 6-TU: 6-thiouric acid; 6-TXMP, 6-thioxanthylic acid; AZA, azathioprine; GMPS, guanosine monophosphate synthetase; HPRT: hypoxanthine-guanine- phosphoribosyltransferase; IMPDH: inosine 5’- monophosphate dehydrogenase; TPMT, thiopurine methyltransferase; XO, xanthine oxidase.
When it comes to the optimal levels of 6-TGN in red blood cells (RBCs), levels of 235–450pmol/8×108 RBCs are considered adequate and associated with remission.8 Lower levels would be insufficient, while levels above 450pmol/8×108 RBCs may increase the risk of myelotoxicity.9 When it comes to 6-MMP, levels of 5700pmol/8×108 RBCs are considered optimal.10
In recent years, a growing number of hospitals have been incorporating thiopurine monitoring in clinical practice. Serum levels of 6-MMP and 6-TGN are measured with the aim of reducing the incidence of myelosuppression, and some studies have reported that it could prevent up to ¼ of myelosuppression episodes6 while also reducing hepatotoxicity10 and improving drug efficacy, increasing the rate of clinical remission.10,11 Other studies suggest that monitoring may also improve adherence to pharmacological treatment.11 However, there have also been studies that did not find a clear improvement with thiopurine monitoring compared to no monitoring.12
The aim of our study was to describe the use of optimised thiopurine therapy in paediatric patients with IBD followed up in our unit from the introduction of thiopurine monitoring, and to assess for the potential benefits derived from monitoring, such as dose adjustment, a decreased risk of toxicity or the optimisation of treatment without need of dose escalation.
Material and methodsStudy sampleWe retrospectively included patients aged 0 to 14 years with an existing diagnosis of IBD managed in our unit that required measurement of thiopurine metabolite levels during the follow-up. The patients had received the drug for at least 12 weeks. The indications for measurement of serum thiopurine metabolite levels were poor or suboptimal control of disease during remission, development of adverse events or concerns regarding adherence.
We reviewed health records to collect demographic and clinical data (disease phenotype, initial presentation, diagnostic workup and disease activity index), and data on laboratory tests (antibodies, complete blood count, C-reactive protein, chemistry panel) and anthropometric measurements. We collected the measured levels of 6-TGN, 6-MMP, the 6-MMP/6-TGN and 6-TGN/6-MMP ratios, TPMT activity, dosage of oral AZA or 6-MP prescribed to the patient, dosage form (most frequently tablets, in some cases solutions, depending on age), concomitant medication and treatment approach after measurement of metabolite levels (dose increase, drug switch, withdrawal of drug or no changes). We defined clinical remission as a weighted Paediatric Crohn's Disease Activity Index (wPCDAI) of less than 12.5 in patients with CD or a Paediatric Ulcerative Colitis Activity Index (PUCAI) of less than 10 in patients with UC.
Measurement of metabolite levelsWe measured serum levels of 6-TGN and 6-MMP (pmol/8×108 RBCs) by means of high-performance liquid chromatography.
Statistical analysisWe conducted a descriptive analysis of the sample and variables under study by calculating the mean, standard deviation, median and interquartile range depending on whether or not the data followed normal distribution. We compared qualitative data by used of the chi square test or Fisher exact test, and quantitative data with the Student t test if the normal distribution and homogeneity of variance assumptions were met, and otherwise the Mann–Whitney U test or the Welch test. We developed prediction models for treatment response by means of univariate and multivariate logistic regression. The variables included in the development of the model were those for which we found statistically significant differences or differences that neared statistical significance in the univariate analysis (P<.15) and variables considered to be associated with the dependent variable based on current knowledge or empirical experience. We developed a multivariate regression model with selection of variables by backward elimination. We measured the strength of the association of the independent variables included in the model and the dependent variable by calculating odds ratios (ORs) with their corresponding 95% confidence intervals. We defined statistical significance as a P-value of less than .05.
Informed consentThe study was approved by the Clinical Research Ethics Committee of the hospital and adhered to the corresponding guidelines. We collected information from health records in adherence to the protocol established by the hospital.
ResultsA total of 140 measurements were made in the 72 patients included in the study. The mean number of measurements per patient was 1.94±1.09. We did not find differences in the baseline characteristics of patients with UC and CD save for the need of biological therapy (more frequent in CD) and the overall treatment used during the followu-p period. Table 1 summarises the characteristics of the patients included in the sample.
Characteristics of included patients.
| Crohn disease | Ulcerative colitis | P | |
|---|---|---|---|
| n | 47 | 25 | |
| Sex (M/F) | 25/22 | 11/14 | .458 |
| Age at diagnosis (mean±SD) | 9.87±3.36 | 8.21±4 | .069 |
| Ethnic background (Mediterranean/Maghrebi) | 41/6 | 20/5 | .417 |
| Anthropometric values at diagnosis (age >5years) (mean±SD) | |||
| Weight (kg) | 36.41±13.4 | 31.26±9.63 | .183 |
| Weight z-score | −0.54±1 | −0.57±0.72 | .298 |
| Height (cm) | 144.72±16.77 | 137.71±17 | .208 |
| Height z-score | −0.17±1.67 | −0.04±1 | .633 |
| BMI (kg/m2) | 17.21±2.86 | 16±2.12 | .107 |
| BMI z-score | −0.72±1.44 | −0.69±0.71 | .228 |
| Family history (Yes/No) | 9/38 | 5/20 | .931 |
| TPMT activity (IU/mL packed RBC) | |||
| <5 | 0 | 0 | |
| 5–13.7 | 4 | 0 | |
| >13.7 | 38 | 17 | |
| Duration of follow-up. years (median/IQR) | 3.95/1.81:3.20–5.01 | 4.30/3.79:2.28–6.07 | .762 |
| Need of biological therapy (Yes/No) | 32/15 | 6/19 | <.001 |
| Time elapsed to initiation of biological therapy in patients receiving monotherapy. months (median/IQR) | 14.16/19.29:7.74–27.03 | 31.57/44.36:1.82–46.17 | .425 |
| Other drugs used during follow-up (number of patients) | |||
| Oral tacrolimus oral | 0 | 5 | .004 |
| Rectal tacrolimus rectal | 1 | 2 | .282 |
| Steroids | 10 | 9 | .159 |
| Enteral nutrition | 19 | 2 | .004 |
| Oral 5-ASA oral | 3 | 17 | <.001 |
| Rectal 5-ASA rectal | 0 | 8 | <.001 |
IQR, interquartile range; RBC, red blood cell; SD, standard deviation; TPMT: thiopurine-methyltransferase.
The dose of AZA used for treatment was adjusted to published guidelines,13 although up to 61.5% of patients had subtherapeutic levels of 6-TGN and 7.4% had levels of 6-MMP in the toxicity range. Table 2 summarises the measured serum metabolite levels. In most cases, the thiopurine dose was increased following the measurement of metabolite levels (Table 3).
Levels of thiopurines expressed in pmol/8×108 RBCs and dose of oral AZA.
| Blood metabolite levels | Median | IQR | Within therapeutic range | Above therapeutic range | Below therapeutic range |
|---|---|---|---|---|---|
| 6-TGN | 211 | 140 (141–281) | 47 (33.8%) | 7 (5%) | 85 (61.5%) |
| 6-MMP | 1321.5 | 2299.39 (490.1–2789.48) | 126 (92.6%) | 10 (7.4%) | |
| 6-MMP/6-TGN | 6.04 | 12.05 (2.67–41.62) | |||
| 6-TGN/6-MMP | 0.15 | 0.30 (0.06–0.36) | |||
| AZA dose (mg/kg/day) | 2.54 | 0.53 (2.25–2.77) |
6-MMP, 6-methylmercaptopurine; 6-TGN, 6-thioguanine; AZA, azathioprine; IQR, interquartile range.
Approach following measurement of thiopurine levels.
| No change | 57 (40.7%) | |
| Change in treatment | 77 (55%) | |
| Dose increase | 62 (45.2%) | |
| Dose decrease | 5 (3.6%) | |
| Discontinuation of drug | 8 (5.8%) | |
| Change in dosage form (switch from solution to tablet or vice versa) | 2 (1.4%) | |
| Initiation of a different drug (methotrexate) | 1 (1.25%) | |
| Initiation of biological therapya | 9/67 (13.4%) |
The logistic regression analysis did not find an association between disease activity/remission and the level of 6-TGN, the daily dose of AZA (in mg/kg or mg/m2) or the 6-TGN/6-MMP and 6-MMP/6-TGN ratios. Table 4 presents the observed metabolite levels in relation to the level of disease activity.
Mean thiopurine metabolite levels measured in pmol/8×108 RBCs in patients in monotherapy by disease activity.
| Plasma metabolites | 6-TGN | 6-MMP | 6-MMP/6-TGN | 6-TGN/6-MMP | |
|---|---|---|---|---|---|
| Patients in remission | Median | 246.08 | 1235 | 5.89 | 0.17 |
| IQR | 228.5 (130.4–358.9) | 3409.55 (581.45–3991) | 12.46 (2.84–15.29) | 0.28 (0.07–0.35) | |
| Patients with active disease | Median | 210.5 | 1321.5 | 7.24 | 0.13 |
| IQR | 124.58 (141.32–265.9) | 1853.34 (702.91–2556.25) | 9.26 (3.42–12.68) | 0.21 (0.07–0.29) |
6-MMP, 6-methylmercaptopurine; 6-TGN, 6-thioguanine; IQR, interquartile range.
Measurement of metabolite levels allowed detection of 4 cases of nonadherence, corresponding to an adherence rate greater than 95%. A new personal and family history was taken in these patients to identify the factors that led to nonadherence.
Adverse eventsIn 17 cases, the patient was receiving an excessive dose (6-TGN >450pmol/8×108 RBCs) or the drug was metabolised via an alternative pathway (6-TGN <230 and 6-MMP >5700pmol/8×108 RBCs). However, there was no evidence of hepatotoxicity or myelotoxicity in any of the patients.
DiscussionOur findings reveal that a high percentage of patients had levels of 6-TGN considered subtherapeutic, while as many as 7.4% had 6-MMP levels in the toxic range at the time of testing.3
A recent study14 found a similar proportion of thiopurine underdosing in the study sample (45.6%), demonstrating that metabolite level monitoring may help identify patients in who increasing the dose of AZA could help maintain remission without addition of other drugs, as recommended by clinical practice guidelines,3,15 as previous studies have described the need for higher-than-standard doses of AZA or 6-MP in some patients to increase the rate of remission.16
Another of the potential advantages of thiopurine monitoring is to prevent drug-related toxicity, as there is evidence that approximately 20% to 305 of patients has to discontinue treatment due to the emergence of adverse effects.17–19 Some forms of hepatotoxicity are associated with 6-MMP levels, suggesting that concentrations of this metabolite greater than 5700pmol/8×108 RBCs are a risk factor for liver toxicity.7,10 In our sample, we found that 10 measurements of 6-MMP (7.4%) detected levels in the hepatotoxicity range, a proportion similar to those reported by other authors.14 As for the levels of 6-TGN, several studies support a positive association with the risk of myelotoxicity,19,20 even in the early stages of treatment,21 and therefore monitoring these levels and making the necessary adjustments could prevent this negative outcome in some cases. In our study, we found that as many as 5% of measurements detected levels greater than recommended, which was similar to the overall proportion of 7% reported in a systematic review published by Gisbert and Gomollón.22
Only 9 of the 67 patients receiving AZA as monotherapy (13.4%) required addition of biological agents to the regimen, a percentage similar to the 12% reported by Suárez et al.23 The mean duration of follow-up of the disease in our unit before initiation of biological therapy was 4.41 years, and the mean duration of monotherapy with AZA was 18 months in patients with CD (the predominant phenotype requiring biological therapy in our case series, with a statistically significant difference in frequency compared to patients with UC). Monitoring thiopurine metabolite levels could potentially reduce escalation to biological therapy or other treatments, although on the other hand biological agents are used increasingly frequently in patients with IBD. In our unit, we found that the number of patients receiving biological therapy was increasing progressively, with a stricter approach in response to the development of certain adverse events associated with thiopurine use. In addition, the safety profile of biological agents is better,24 and there is evidence that biological therapy, alone or in combination, can achieve mucosal healing in a higher percentage of patients.25,26
However, we believe that from an economic standpoint thiopurine monitoring may be cost-effective, a factor previously evaluated by other authors,27 partly due to the lower cost of thiopurines compared to other treatment options such as biological drugs. However, more cost-effectiveness studies are necessary to be able to make clear recommendations in this regard.
In agreement with previous studies in adults,28,29 and contrary to the findings reported by other authors,8,30,31 we did not find an association between 6-TGN and disease activity. This was probably due to the high number of cases lost to follow-up, which can be attributed to the retrospective data collection, and to the absence of wPCDAI or PUCAI scores recorded at the time of the appointment, which in most cases was due to other laboratory tests used in assessing disease activity not being performed on the same date that samples were collected for measurement of metabolite levels. The baseline characteristics of the population under study must also be taken into account when interpreting results, as they may limit external validity. For instance, in the study conducted by Lee et al.,30 thiopurine metabolite levels were measured in patients receiving azathioprine in combination with mesalamine and not in patients receiving AZA as monotherapy. In addition, the mean dose of AZA in that study was considerably lower compared to the mean dose received by patients in our unit (1.01mg/kg/day versus 2.54mg/kg/day), which may have had an impact on the results, as higher doses would be more strongly associated with remission based on various guidelines that recommend administration of more than 2mg/kg/day of AZA in patients with IBD3 and normal TPMT activity. Another possible explanation would be that patients in the study by Lee et al. were Asian, whereas most patients in our unit were Caucasian or of Maghrebi descent, and the levels of AZA required to maintain remission may vary based on racial and ethnic background.32 A systematic review by Konidari et al.10 led to the conclusion that thiopurine levels cannot be used to predict clinical remission but can help optimise treatment and prevent drug toxicity, and a recent meta-analysis found association between 6-TGN levels and the rate of remission and attributed the conflicting results of previous studies to the lack of standardisation in thiopurine level assays.33
Our study has limitations, chief of which is its retrospective design. On the other hand, the large number of tests and the substantial patient follow-up are two of its main strengths, and our study is relevant given the lack of similar studies in Spain.
Based on the experience in our unit, it is reasonable to conclude that thiopurine metabolite monitoring in paediatric patients with IBD is effective for the purpose of guiding the therapeutic approach, as it may help optimise thiopurine therapy and in many cases prevent escalation to other treatments, usually biological therapy. It may also be useful for detection of high drug concentrations that may cause toxicity, the prevention of which also makes this intervention useful for the purpose of treatment optimisation in these patients. We believe that thiopurine monitoring should be used in most hospitals. Further research is needed to clearly establish the percentage of patients that benefit from monitoring and the circumstances in which it is indicated, in addition to the use of pharmacogenetic testing to determine individual risk in patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martín-Masot R, Ortiz Pérez MP, Ramos Rueda N, Serrano Nieto J, Blasco-Alonso J, Navas-López VM. Análisis de la determinación de niveles de tiopurínicos en pacientes pediátricos con enfermedad inflamatoria intestinal. An Pediatr (Barc). 2020;93:34–40.