Nonbacterial osteomyelitis (NBO) is an autoinflammatory disease that manifests with pain, swelling and/or functional limitation due to inflammation at one or more bone sites, although it is sometimes asymptomatic, and may be acute (<2 weeks) or chronic (>2 weeks). Chronic recurrent multifocal osteomyelitis, the most severe form, manifests with chronic inflammation lasting more than 6 months. The laboratory findings are nonspecific, with mild or no elevation of acute phase reactants. Although a bone scan may be useful to localise active asymptomatic sites, whole body magnetic resonance imaging (MRI) is the test of choice, as it can evince the presence of bone swelling, osteolysis, hyperostosis or sclerosis, which are characteristic features. It is a diagnosis of exclusion based on a combination of clinical, radiological and anatomical/histological findings, based on the Jansson1 or Bristol2 diagnostic criteria. Performance of a bone biopsy is particularly indicated in cases with unifocal involvement, of short duration and presenting with osteolysis, as it allows ruling out malignant and infectious disease.
The first-line treatment consists of nonsteroidal anti-inflammatory drugs (NSAIDs), alone or combined with steroid therapy, and there is no consensus regarding the second-line treatment. Different synthetic disease-modifying antirheumatic drugs, biologics such as tumour necrosis factor alpha (TNFα) inhibitors or bisphosphonates (especially in the case of spinal involvement) are used, with significant variability in clinical practice. Of the available bisphosphonates, the Childhood Arthritis and Rheumatology Research Alliance (CARRA) consensus3 proposes both pamidronate and zoledronate as acceptable options. Although the dosage of zoledronate is more convenient, few studies have assessed its use (Table 1).
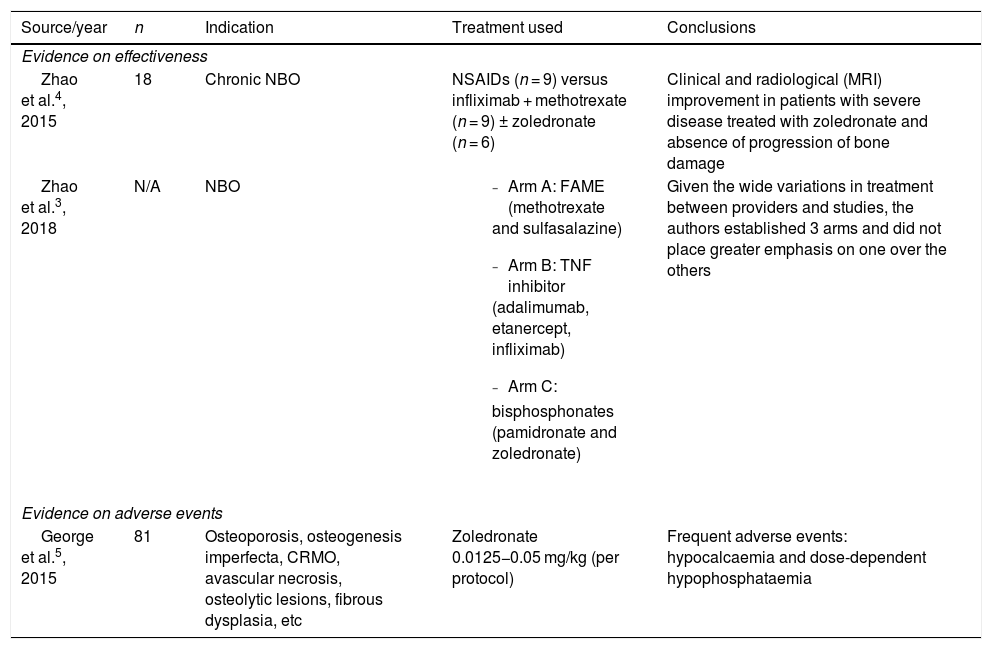
Review of the literature about the use of zoledronate for NBO.
| Source/year | n | Indication | Treatment used | Conclusions |
|---|---|---|---|---|
| Evidence on effectiveness | ||||
| Zhao et al.4, 2015 | 18 | Chronic NBO | NSAIDs (n = 9) versus infliximab + methotrexate (n = 9) ± zoledronate (n = 6) | Clinical and radiological (MRI) improvement in patients with severe disease treated with zoledronate and absence of progression of bone damage |
| Zhao et al.3, 2018 | N/A | NBO |
| Given the wide variations in treatment between providers and studies, the authors established 3 arms and did not place greater emphasis on one over the others |
| Evidence on adverse events | ||||
| George et al.5, 2015 | 81 | Osteoporosis, osteogenesis imperfecta, CRMO, avascular necrosis, osteolytic lesions, fibrous dysplasia, etc | Zoledronate 0.0125−0.05 mg/kg (per protocol) | Frequent adverse events: hypocalcaemia and dose-dependent hypophosphataemia |
CRMO, chronic recurrent multifocal osteomyelitis; NBO, nonbacterial osteomyelitis; NSAID, nonsteroidal anti-inflammatory drug.
Given the above, we conducted a retrospective, observational, descriptive and analytical study in patients aged less than 16 years with a diagnosis of NBO based on the Jansson criteria, managed in a tertiary care hospital and who received bisphosphonates as the first choice for second-line therapy in the 2013–2020 period, with the aim of comparing the effectiveness and safety of both treatments. We analysed clinical, laboratory and radiological variables. The dose of pamidronate was 1 mg/kg/month, with the first dose administered over 3 consecutive days (day 1: 0.5 mg/kg; days 2 and 3: 1 mg/kg/day) compared to a dose of zoledronate of 0.025 mg/kg every 3 months. After 2 doses of treatment, we defined complete response as absence of pain and fever with normalization of laboratory and radiological features, partial response as clinical improvement not meeting criteria for complete response, and no response as absence of improvement. We considered adverse events any side effects reported by the patients, changes in electrolytes or nephrocalcinosis.
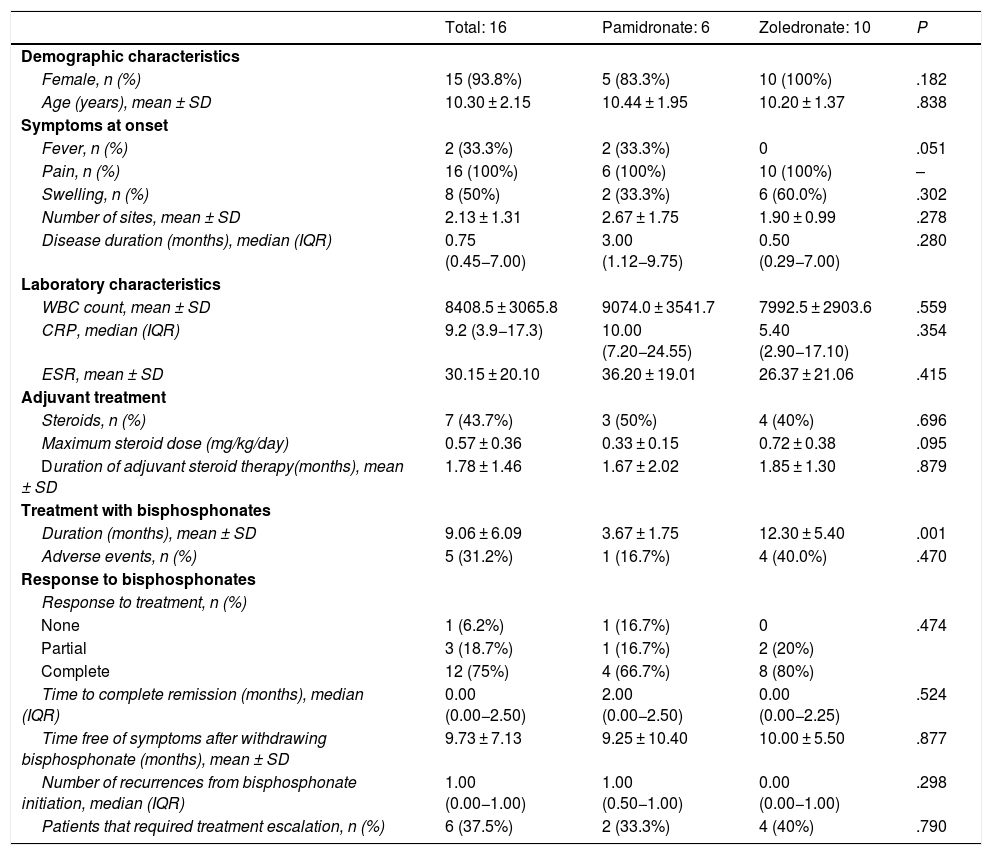
We collected data on 16 treatment courses in 12 patients, 6 treated with pamidronate and 10 with zoledronate. We did not find statistically significant differences between both groups in sex, age, laboratory characteristics at baseline or the need of oral steroid therapy to achieve remission. None of the patients treated with zoledronate had fever. The mean duration of treatment with pamidronate was 3.67 months (standard deviation [SD], 1.75) compared to 12.30 months with zoledronate (SD, 5.40) (P = .01). In the pamidronate group, 66.7% of patients achieved a complete response, compared to 80% in the zoledronate group (P = .474). The median number of recurrences was 1 with pamidronate (interquartile range [IQR], 0–2.5) compared to 0 with zoledronate (IQR, 0–2.25) (P = .298). Escalation to treatment with a TNF inhibitor (adalimumab) due to nonresponse was required in 33.3% of patients treated with pamidronate and 40% of patients treated with zoledronate (P = .79). The only observed adverse event was flu-like syndrome, which developed in 16.7% of patients treated with pamidronate compared to 40% of those treated with zoledronate (P = .470) and was self-limited in every case (Table 2).
Bivariate analysis comparing demographic, clinical, laboratory and treatment-related characteristics in both groups.
| Total: 16 | Pamidronate: 6 | Zoledronate: 10 | P | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Female, n (%) | 15 (93.8%) | 5 (83.3%) | 10 (100%) | .182 |
| Age (years), mean ± SD | 10.30 ± 2.15 | 10.44 ± 1.95 | 10.20 ± 1.37 | .838 |
| Symptoms at onset | ||||
| Fever, n (%) | 2 (33.3%) | 2 (33.3%) | 0 | .051 |
| Pain, n (%) | 16 (100%) | 6 (100%) | 10 (100%) | – |
| Swelling, n (%) | 8 (50%) | 2 (33.3%) | 6 (60.0%) | .302 |
| Number of sites, mean ± SD | 2.13 ± 1.31 | 2.67 ± 1.75 | 1.90 ± 0.99 | .278 |
| Disease duration (months), median (IQR) | 0.75 (0.45−7.00) | 3.00 (1.12−9.75) | 0.50 (0.29−7.00) | .280 |
| Laboratory characteristics | ||||
| WBC count, mean ± SD | 8408.5 ± 3065.8 | 9074.0 ± 3541.7 | 7992.5 ± 2903.6 | .559 |
| CRP, median (IQR) | 9.2 (3.9−17.3) | 10.00 (7.20−24.55) | 5.40 (2.90−17.10) | .354 |
| ESR, mean ± SD | 30.15 ± 20.10 | 36.20 ± 19.01 | 26.37 ± 21.06 | .415 |
| Adjuvant treatment | ||||
| Steroids, n (%) | 7 (43.7%) | 3 (50%) | 4 (40%) | .696 |
| Maximum steroid dose (mg/kg/day) | 0.57 ± 0.36 | 0.33 ± 0.15 | 0.72 ± 0.38 | .095 |
| Duration of adjuvant steroid therapy(months), mean ± SD | 1.78 ± 1.46 | 1.67 ± 2.02 | 1.85 ± 1.30 | .879 |
| Treatment with bisphosphonates | ||||
| Duration (months), mean ± SD | 9.06 ± 6.09 | 3.67 ± 1.75 | 12.30 ± 5.40 | .001 |
| Adverse events, n (%) | 5 (31.2%) | 1 (16.7%) | 4 (40.0%) | .470 |
| Response to bisphosphonates | ||||
| Response to treatment, n (%) | ||||
| None | 1 (6.2%) | 1 (16.7%) | 0 | .474 |
| Partial | 3 (18.7%) | 1 (16.7%) | 2 (20%) | |
| Complete | 12 (75%) | 4 (66.7%) | 8 (80%) | |
| Time to complete remission (months), median (IQR) | 0.00 (0.00−2.50) | 2.00 (0.00−2.50) | 0.00 (0.00−2.25) | .524 |
| Time free of symptoms after withdrawing bisphosphonate (months), mean ± SD | 9.73 ± 7.13 | 9.25 ± 10.40 | 10.00 ± 5.50 | .877 |
| Number of recurrences from bisphosphonate initiation, median (IQR) | 1.00 (0.00−1.00) | 1.00 (0.50−1.00) | 0.00 (0.00−1.00) | .298 |
| Patients that required treatment escalation, n (%) | 6 (37.5%) | 2 (33.3%) | 4 (40%) | .790 |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell.
Few studies in the literature have compared the effectiveness and safety of different bisphosphonates and of bisphosphonates versus other possible treatments in children with NBO. The study published by Zhao et al.4 is the only one that assessed the differences in clinical and radiological outcomes in 9 patients treated with infliximab + methotrexate, of who 6 also received zoledronate, and 9 treated with NSAID monotherapy, finding improvement in every endpoint only in the first group. In our study, we analysed the number of patients who responded to treatment, and found no differences between groups.
Of the few adverse events described in association with the use of zoledronate, the main ones are flu-like syndrome and changes in phosphate and calcium metabolism (hypocalcaemia/hypophosphataemia)5.
The only adverse event observed in our series was flu-like syndrome, mild and only following the initial infusion. The absence of other adverse events was probably due to the calcium and vitamin D supplementation that the patients received before and after each infusion.
In conclusion, the use of zoledronate compared to pamidronate for treatment of NBO seems equally effective and safe while offering a more convenient dosage, shorter hospital stays and possibly an improved quality of life to these patients. On account of these advantages, it could be contemplated as the bisphosphonate of choice in patients with NBO. However, this was a single centre, retrospective study in a small sample, so multicentre studies are required to corroborate these findings.
Previous presentation: this study was presented at the XIV Congress of the Sociedad Española de Reumatología Pediátrica (SERPE), held online on November 25 and 26, 2021, an the 68th Congress of the Asociación Española de Pediatría (AEP), held on June 2–4, 2022 in Palma de Mallorca, Spain.