Why the Tenth Symphony of Gustav Mahler was Unfinished?
Gustav Mahler 1,2 was born on July 7, 1860, in Kalista (Bohemia) near Moravia. His first musical work was a polka composed at the age of 6 years. Mahler studied music at the Vienna Conservatory. He was a composer much criticized by the press. In 1902, Mahler married Alma Schindler, with whom he had 2 daughters, Anna and Maria. The latter died of scarlet fever at the age of only 5. The death of his younger daughter left him depressed; that same year, he discovered he had a heart disease. Forced by a largely anti-Semitic press, he accepted an offer to conduct the Metropolitan Opera in New York in 1907. In 1911, he fell seriously ill with endocarditis. He was attended by Dr. E. Libman who demonstrated the presence of Streptococcus viridans in a large volume (200 ml) of his blood. Mahler was taken to Paris and treated with "Metchnikoff's Bulgarian milk" (Lactobacillus bulgaricus), the probiotics of that era. However, septic abscesses began to appear in other parts of his body. He was taken back to Vienna, and died on May 18, 1911, leaving his Tenth Symphony incomplete.
Introduction
Infectious endocarditis is a major infection involving the endocardium, particularly the cardiac valves. For a long time, it was called bacterial endocarditis. Actually, in addition to bacteria, infectious endocarditis can be caused by other microbiological agents. Changes in the presentation of this disease are explained by modifications in susceptible populations, predisposing factors, and the evolution of microorganisms. Despite a great deal of progress made in recent decades, the diagnosis and treatment of endocarditis continue to be difficult.
Historical aspects
Fernel's Medicini in 1554 was the first book to introduce the term of endocarditis 3. During the 17th and 18th centuries, anomalies of the cardiac valves were described during autopsies of these patients 2. In 1669, Richard Lower in England was the first to diagnose endocarditis of the tricuspid valve. In 1806, Jean Nicolas Corvisart (1755-1821) was probably the first to use the term "vegetations" 2. In 1816, Théophile Laënnec 1 invented the cylindrical stethoscope, improving cardiac auscultation. In 1835, Jean-Baptiste Bouillaud 2 defined the endocardium in his "Traité clinique des maladies du coeur". In France, routine blood cultures were introduced by Pasteur in the late 19th century 1. Penicillin was discovered by Sir Alexander Fleming in 1929 and it has being administered for the treatment of this disease since 1940 2.
Epidemiology
Currently, the incidence of endocarditis is 1-4 cases/100,000 or 1/1,300 annual paediatric admissions 4,5. The increased occurrence of endocarditis is related to improved survival of children with congenital heart disease, newborns or other very ill children. Vascular conduits, patches or valvuloplasty in children with congenital heart disease are risk factors for endocarditis 6. Other risks are: catheter use in critically ill children, children with immunodeficiency, and the neonatology and the paediatric intensive care units 7. Eight to 10 percent of paediatric infectious endocarditis cases occur in healthy hearts. Endocarditis caused by intravenous drug use is rare in paediatrics. In the past, rheumatic fever was a risk factor, but it has disappeared in the western world. The epidemiology of endocarditis also has changed, thanks to the development and evolution of paediatric cardiology.
Pathogenesis
An injury in the endotelium is the first inducer of thrombogenesis 4, allowing bacteria to adhere and form vegetation. In children with cardiac malformations and turbulent or abnormal blood flow, injuries to the endothelium can easily arise. Catheters traumatize the endocardium 8. Cutaneous or mucosal injuries from tracheal suction, parenteral feeding and umbilical or peripheral catheters are at the origins of bacteraemia in newborns 4. Neonatal endocarditis frequently affects the right heart of newborns. If there is a critical mass of bacteria in the blood during bacteraemia, they can propagate and adhere to the endocardium. During thrombogenesis, blood platelets, sanguinous fibrin and blood cells collect as deposits, and an aseptic thrombus is formed. Bacteria colonize the aseptic thrombus, and blood platelets, fibrin and blood cells are deposited over these organisms, creating vegetation. Microorganisms trapped in the vegetation are protected from phagocytes and other defence mechanisms 4.
Clinical manifestations
The presentation can be insidious with prolonged fever and non-specific symptoms: fatigue, weakness, anorexia, weight loss, and sweating. At other times, it can be sudden, and these children are terribly ill. Endocarditis presents 4 phenomena:
1.Bacteraemia or fungemia.
2.Valvulitis: new heart murmur or cardiac insufficiency.
3.Immunological responses, much less frequent in children than in adults: petechias, haemorrhages, injuries of Roth or Janeway, Osler nodules or splenomegaly.
4.Embolic phenomena may involve the kidneys, abdominal viscera, brain or heart.
In newborns, the presentation is non-specific. Septic emboli are frequent, causing: osteomyelitis, meningitis or pneumonia 4.
Etiology and Laboratory data
Blood culture is indicated in all children with fever of unknown origin, pathological murmur, a history of cardiac malformation or antecedents of endocarditis. During endocarditis, bacteraemia is continuous; therefore, blood cultures can be done at any time 9. It is important to collect an adequate amount of blood; in small children, this can vary from 1 to 3 ml, and in older children, 5-7 ml. Three blood cultures 4,5 detect more than 95 % of endocarditis in children not exposed to antibiotics, and 90 % in children who have received antibiotics 10-13.
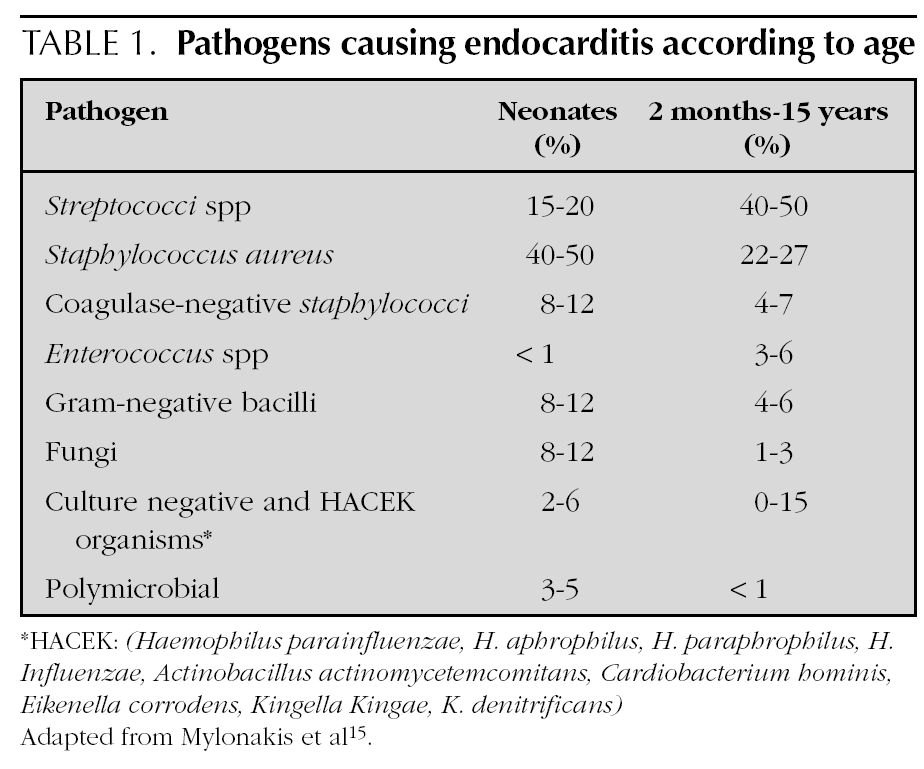
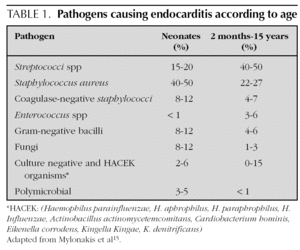
Most agents which cause endocarditis are gram-positive cocci 14: Streptococci, Staphylococci, Enterococci. The organisms most frequently detected in infectious endocarditis are the S. viridans and the S. aureus4. In endocarditis caused by catheters, the S. aureus and the Staphylococci coagulase-negative are frequent. In addition to those 2 agents, it is necessary in newborns to include the presence of Candida, Klebsiella and Enterobacter14. The organisms classified as the HACEK group are less frequently found at a paediatric age: Haemophilus parainfluenzae, H. aphrophilus, H. paraphrophilus, H. influenzae, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella Kingae and K. denitrificans4. Fungi are represented by Candida and Aspergillum. Mylonakis et al 15 enumerated the percentage of microbiological agents detected according to patient age (table 1).
Endocarditis is diagnosed despite negative blood culture if clinical symptoms and heart ultrasound show evident signs of infection. The incidence of negative blood culture is approximately 5-7 %, mostly occurring in patients taking antibiotics or in endocarditis produced by pathogens other than bacteria 4,16. Vegetation culture can help in the diagnosis 16. Other laboratory results are non-specific: anaemia, leucocytosis, abnormal sedimentation rate, protein C-reactivity, hyper-gammaglobulinaemia, haematuria, proteinuria.
Echocardiography
For more than 15 years, heart ultrasound has been revolutionizing the diagnosis of endocarditis. This technique visualizes the place of infection, the vegetation, the extent of injury to the valves, and cardiac function. It also evaluates disease severity and influences medical or surgical treatment decisions. Doppler ultrasound allows the diagnosis of stenosis and valve insufficiency. Transthoracic echocardiography, with a sensitivity of 81 %, is very helpful in the diagnosis of paediatric endocarditis 16. Transoesophagic echocardiograpahy is employed less often in children, and frequently if transthoracic echocardiography is incapable of detecting vegetation 17-19. However, the absence of vegetation does not exclude the diagnosis of endocarditis. Endocarditis affects the valves, but it can also be located in a defect of the septum, the tendinous cords or wall of the endocardium.
The diagnosis of endocarditis is difficult 20 as its clinical manifestations are numerous and non-specific. This explains why the differential diagnosis of endocarditis is important. Considering the consequences of misdiagnosed endocarditis, false-positives do occur. In countries with high immigration rates, it is necessary to remember that recurrence of rheumatic fever can present with the same clinics as endocarditis 21,22. Echocardiography in conjunction with clinical suspicion is the best criterion for the diagnosis of endocarditis.
Criteria for diagnosis
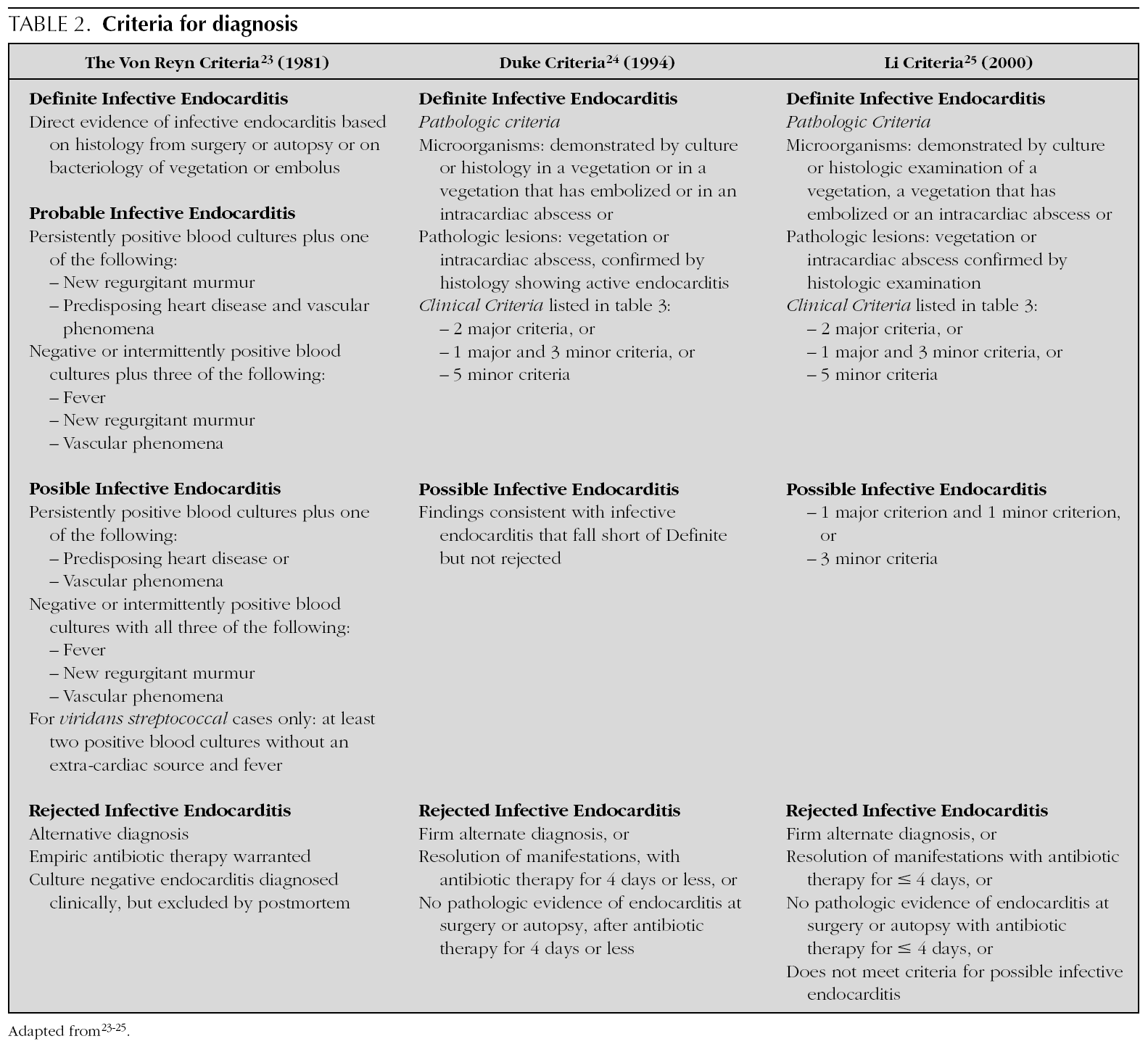
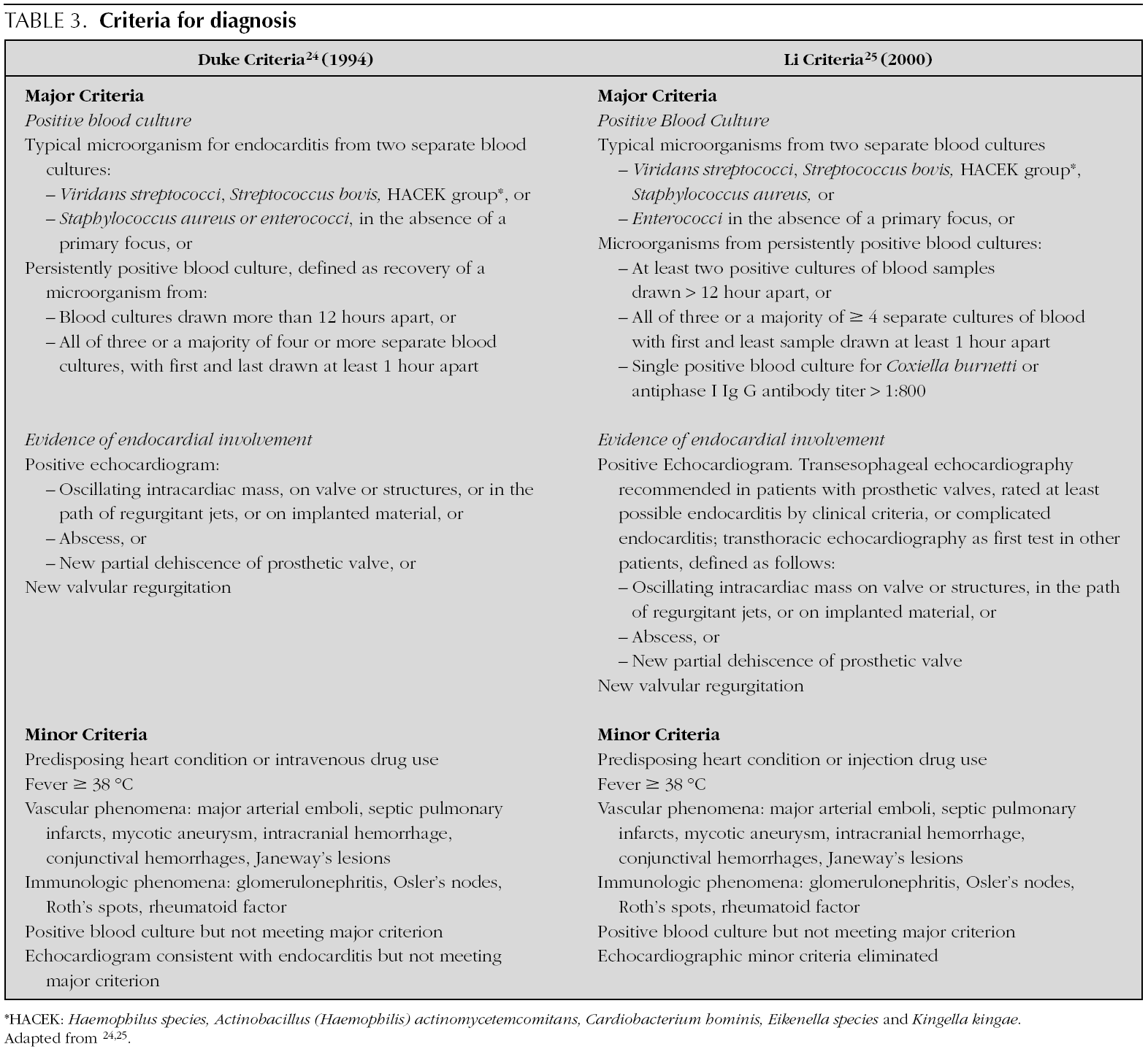
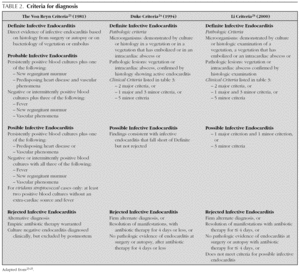
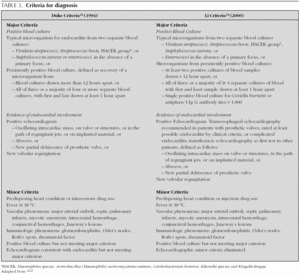
In 1909, Sir Thomas Horder 1 from England described the first criteria for diagnosis: the signs and symptoms of endocarditis. Throughout the 20th century, these criteria have undergone various modifications. In 1981, Von Reyn et al 23 (table 2) proposed criteria to facilitate the diagnosis of the disease. These authors presented 123 adults treated at the Beth Israel Hospital in Boston and classified endocarditis as: definitive, probable, possible, or rejected. In 1994, at Duke University in North Carolina, Durack et al 24 (tables 2 and 3) proposed new diagnostic criteria with the introduction of echocardiography: definitive, possible or rejected endocarditis. According to the criteria of Durack 24 et al, any case of endocarditis not rejected must be considered possible. These criteria seem to present good sensitivity, but are non-specific. In 2000, Li et al 25 (tables 2 and 3) tried to be stricter in endocarditis diagnosis and modified the earlier criteria of Durack 24 et al.
The above-mentioned diagnostic criteria have been validated for use in adult cardiology 26. Some authors have tried to validate these criteria in paediatric cardiology 27-29. However, infectious endocarditis is a disease with a very variable clinical presentation. Isolated criteria are not sufficient to make a diagnosis. The various criteria are clinical guides to help in the diagnosis, but they do not replace clinical judgement. Molecular diagnosis can be helpful in cases with negative blood culture 30. Echocardiography also has been very useful. In the paediatric population, transthoracic echocardiography gives considerable information; transoesophagic echocardiography is rarely necesary 17-19. Echocardiography is not indicated if there is no clinical evidence to support the diagnosis of endocarditis. Each patient with suspicion of endocarditis deserves critical evaluation, to improve the clinical-microbiological diagnosis and treatment 31.
Treatment
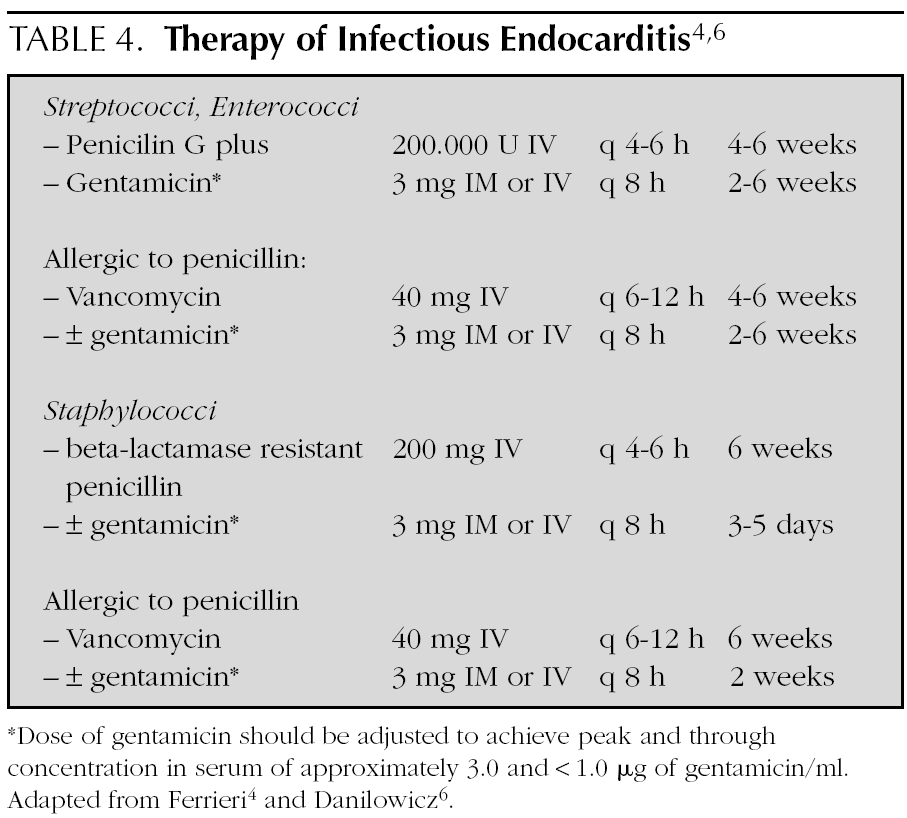
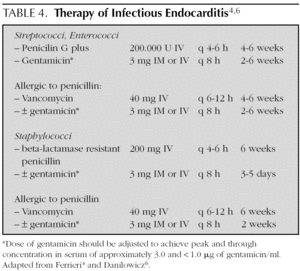
In general, treatment is given for 4 weeks, but is extended up to 6 weeks if the symptoms of presentation have lasted more than 3 months (table 4) 4,6,15,32. Treatment is initiated in the hospital, but in some cases, it is complemented with antibiotic therapy at home.
Complications and outcome
The clinical situations that favour complications 4,31,32 and surgical indications according to the anomalies found at echocardiography are listed in table 5. Coward et al 33 reported a 49 % incidence of complications. Mylonakis et al 15 found 20-25 % adult mortality secondary to endocarditis. Danilowicz 6 recorded a 20-30 % incidence of mortality in paediatric age patients, but recently some authors 33 have determined the incidence to be 12 %.
Prevention
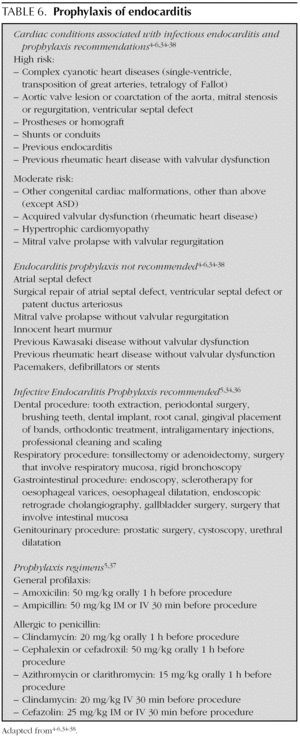
The indications and doses of antibiotics to prevent infectious endocarditis are listed in table 6 4-6,34-38.
Conclusion
1.Paediatric infectious endocarditis is rare, but its incidence has risen owing to the survival of children with operated congenital heart disease.
2.In recent decades, the paediatric population at risk of endocarditis has changed, given the increase of children with immunodeficiency disease and children under neonatal and paediatric intensive care.
3.Infectious endocarditis in healthy children is rare but not exceptional.
4.Complications continue to be frequent.
5.Diagnostic criteria are guidelines that do not replace clinical judgement.