Bloodstream infections (BSIs) are the most frequent nosocomial infections in neonatal intensive care units (NICUs), especially in very low birth weight (VLBW) infants (birth weight ≤ 1500g). An epidemiologic surveillance system may contribute to the prevention of infection by continuous monitoring of its frequency and associated risk factors. The aim of this article was to describe the implementation of the NeoKissEs surveillance system for BSIs in VLBW newborns in a group of Spanish NICUs.
MethodsWe assessed the clinical cohort consisting of all VLBW newborns aged less than 28 days admitted to the participating units. In the pilot phase, 2NICUs translated and adapted materials from the original German NEO-KISS system. During implementation, 210 health care professionals attended one of 8 educational workshops. A web-based system was created that allows entering data regarding patients and BSI episodes, data monitoring, benchmarking and providing feedback to the units. At each NICU, one neonatologist was responsible for the implementation of the system and reporting the difficulties perceived throughout the process.
ResultsOut of the 50 units that agreed to participate, 45 successfully started using the surveillance platform during the implementation phase, recording 1108 episodes of catheter-associated BSI (CABSI) in 3638 newborns, and finding an overall rate of CABSI of 18.4 (95% CI, 17.8-19.1) per 1000 catheter days.
ConclusionsThe NeoKissEs surveillance system constitutes a helpful source of information for the purpose of benchmarking the performance of neonatal units, assessing factors associated with BSI in VLBW infants and measuring the impact of future preventive interventions in NICUs.
Las sepsis son las infecciones nosocomiales más frecuentes en las Unidades de Cuidados Intensivos Neonatales (UCIN), afectando especialmente a los recién nacidos de muy bajo peso al nacer (RNMBP, ≤ 1.500g). Un sistema de vigilancia epidemiológica puede contribuir a su prevención mediante una evaluación continua de su frecuencia y factores de riesgo asociados. El objetivo de este artículo es describir la implementación del sistema de vigilancia de las sepsis nosocomiales en RNMBP (NeoKissEs) en un grupo de UCIN españolas.
MétodosEstudio de cohorte de RNMBP con < 28 días de edad ingresados en las UCIN participantes. Dos UCIN tradujeron y adaptaron materiales a partir del sistema original alemán NEO-KISS. Durante la implementación, se desarrollaron 8talleres formativos, con participación de 210 profesionales. Se creó un sistema web para la introducción de datos de pacientes y episodios de sepsis, su monitorización, análisis comparativo y retroalimentación a las unidades. En cada UCIN, un neonatólogo fue responsable de la implementación, recogiendo información sobre las dificultades percibidas durante el proceso.
ResultadosDe 50 unidades que aceptaron participar, 45 utilizaron NeoKissEs durante la fase de implementación, registrando 1.108 episodios de sepsis asociados a catéter vascular en 3.638 neonatos, con una tasa de 18,4 episodios por 1.000 pacientes-día con catéter (IC del 95%: 17,8-19,1).
ConclusionesEl sistema de vigilancia epidemiológica NeoKissEs representa una fuente útil de información para la comparación estandarizada de la incidencia de sepsis de las UCIN, evaluar factores de riesgo y facilitar la evaluación del efecto de futuras intervenciones preventivas.
Very low birth weight (VLBW) infants (birth weight ≤ 1500g) are particularly vulnerable to nosocomial infections. The immaturity of their immune system, the fragility of their skin and the multiple life support procedures used in their care are among the factors associated with the high incidence of these infections and the associated morbidity and mortality.1,2 Bloodstream infections (BSIs) are the most frequent nosocomial infections in neonatal intensive care units (NICUs). Epidemiologic surveillance systems may contribute to the prevention of infection through the continuous assessment of its incidence and the associated risk factors.3–5 Such systems must regularly produce standardised indicators for self-assessment of health care quality and benchmarking among participating NICUs.6–8 In addition, the implementation and management of a surveillance system requires a sound methodology and an effective organisational strategy to obtain accurate and reliable data on an ongoing basis for the purpose of assessing the impact of newly introduced preventive and quality improvement measures.9,10
In Europe, the German National Reference Center for Surveillance of Nosocomial Infections, which has implemented a specific system for VLBW infants (NEO-KISS), has pioneered the epidemiological surveillance of BSI.5 A decrease in the incidence of BSI was observed in the early years of operation of this system in Germany.4 During this time, there was no nationwide system for prospective data collection that would have allowed Spanish NICUs to document and track BSIs in VLBW infants. This posed challenges to the standardised measurement of incidence rates and of associated risk factors, especially those that are actually modifiable (catheter and antibiotic use).
The purpose of this article was to describe our experience in the implementation of the Spanish NeoKissEs system for the surveillance of BSIs in VLBW infants. NeoKissEs is based on the German NEO-KISS, whose definitions and methodology have been validated and are currently applied in some of the surveillance protocols of the European Centre for Disease Prevention and Control.11
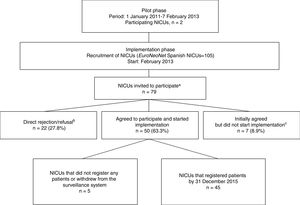
MethodsProtocol development and NICU recruitmentCruces University Hospital and forms used in the German NEO-KISS system to local needs.5,12 A coordination team of health care professionals in the fields of neonatology, preventive medicine and epidemiology was established. Later, 12 de Octubre Hospital Universitario (Madrid) joined the pilot phase, and the two hospitals collaborated in developing the full adapted surveillance protocol in close communication with the staff of the German NEO-KISS system. A structured process for the voluntary enrolment of NICUs nationwide was initiated in February 2013 (Figure 1).
Recruitment of neonatal intensive care units (NICUs) eligible for implementation of the NeoKissEs surveillance system.
a 26 of the 105 NICUs that registered patients in EuroNeoNet did not meet inclusion criteria.
b 4 NICUs declined the invitation and 18 did not respond. These 22 NICUs were located in 9 of the 17 Spanish administrative regions, their median number of deliveries per year was lower compared to participating NICUs (1925 vs 2704; P =.011), they tended to be smaller (fewer admissions and fewer both intensive and medium care cots) and they provided less complex care.
c 2 NICUs reported admitting less than 15 VLBW infants per year, 1 did not receive approval from their Clinical Research Ethics Committee and 4 did not start implementing the system.
The inclusion criteria were: having at least 15 admissions of VLBW infants annually and a documented incidence of BSI above the first quartile for the reference population (infants admitted to Spanish NICUs that were part of the European Neonatology Network [EuroNeoNet] between 2006 and 2011).13
Seventy-nine Spanish NICUs of the 105 registered with EuroNeoNet fulfilled the inclusion criteria and were invited to participate (Figure 1). All participating NICUs provided information on the size and characteristics of their catchment population and their unit. This information was used to categorise the units based on their level of care according to the definitions of the Standards Committee of the Sociedad Española de Neonatología (Spanish Society for Neonatology).14
Extending the system to other hospitalsThe NeoKissEs protocol was reviewed by all participating hospitals and evaluated and approved by their respective Clinical Research Ethics Committees, which waived the written informed consent requirement. Nevertheless, parents and legal guardians were informed about the surveillance system.
A neonatologist was appointed in each NICU to oversee the implementation of NeoKissEs. These neonatologist champions participated in a workshop that introduced them to the protocol, its application and the use of associated instruments (manuals, video tutorials, the electronic case report form and statistical reports). All these instruments were available at the system's website (www.neokisses.com), which was created to register patients and episodes of BSI and to which only the appointed champions had password-protected access.
The coordination team recommended that the implementation team in each NICU was formed by at least 2 staff from the unit: a nurse with experience in neonatal care and a senior neonatologist. One of them would be responsible for the daily collection of data (catheter and antibiotic use), and the other with verifying the validity and accuracy of the data and the fulfilment of the NeoKissEs criteria for the definition of an episode of BSI before entering the recorded data into the system. The coordination team also recommended integrating staff with experience in infection control (usually employed in preventive medicine departments).
In mid-2014, a survey was carried out among the 45 NICUs that had initiated the implementation of NeoKissEs, asking about the difficulties experienced by the champions and their teams in the process, and the strategies used to address them.
Data collectionThe target population of NeoKissEs comprises all VLBW infants admitted to a participating NICU at age less than 28 days, regardless of gestational age at birth. Every VLBW infant registered in NeoKissEs was followed up until discharge from the unit, transfer to another hospital, death or reaching of a body weight of 1800g, in accordance with the NEO-KISS criteria.
At NICU admission, demographic, clinical and perinatal data are recorded in what is called the patient surveillance master data form (with fields for birth weight, gestational age, date of birth and of admission, type of delivery, sex, number of foetuses and Clinical Risk Index for Babies [CRIB] score).15 When the required fields are filled out, the system assigns a surveillance code number to the infant and the form becomes an active record in the surveillance programme. From this moment, the team member assigned to the task (usually a nurse) fills out a paper-based patient progress chart every day, checking whether the care of the patient includes any of the following interventions: placement of a central or peripheral vascular catheter, and antibiotic administration. A cumulative summary is produced for each patient at the end of each month. When the period of surveillance ends for a patient, the data from their progress charts are entered manually into the online database as a cumulative summary of the data collected monthly. The facilitator of the NICU assesses and guarantees the quality of the comprehensive data entered in the system.
NeoKissEs outcomesThe primary outcome of NeoKissEs is primary BSI, understood as BSI with onset at least 72hours after birth or admission of an eligible infant to the NICU.5,16 The NeoKissEs protocol adheres strictly to the German NEO-KISS definitions of BSI, which were originally adapted from those of the Centers for Disease Control and Prevention and have been tested and validated in German NICUs.12,17
When a facilitator detects a possible episode of primary BSI (alerted by the staff managing the patient or by the initiation of a course of antibiotic treatment), they open a BSI data collection form, which contains a checklist of the signs and symptoms required for a clinically suspected BSI episode to qualify as a NeoKissEs BSI event. At this point, the system requests information about culture results, which are used to classify the current BSI episode as one of the following:
- -
Microbiologically-confirmed BSI with coagulase-negative Staphylococcus as the sole pathogen.
- -
Microbiologically-confirmed BSI with detection of at least one pathogen other than coagulase-negative Staphylococcus.
- -
Clinical BSI, with no pathogen isolated in culture but in which the patient receives antimicrobial treatment for at least 5 days.5
The online system does not register the episode into the BSI database unless all required fields have been filled out, and the neonatologist appointed as facilitator has to verify the accuracy of all BSI-related data. The coordination team reviews all new episodes and sends queries to the facilitator if it finds any inconsistencies.
NeoKissEs automatically calculates statistics, including the incidence density of BSI per 1000 patient days, the rate of catheter-associated bloodstream infection (CABSI) and the frequency of use of interventions such as catheterization and antibiotherapy.
Data processing and disseminationThere are two ways in which data are analysed and feedback provided to participating NICUs. First, for each NICU, the online system can generate real-time statistical reports of outcomes on demand, overall and by birth weight and gestational age subsets. In addition, when the data for every case included in a calendar year is complete, the coordination team produces an annual report. This report offers more detailed information adjusted for birth weight, gestational age and level of care, and excludes data from VLBW infants who were transferred, discharged or deceased within 72hours of NICU admission. It provides a detailed account of surveillance indicators for each NICU as well as two benchmarking summaries: one based on the values observed in the Spanish reference population of NeoKissEs, and another based on the overall statistics for the same year in the German NEO-KISS population.
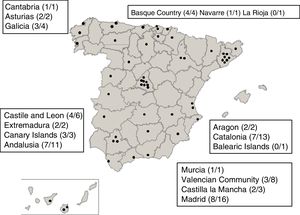
ResultsThe implementation of NeoKissEs started with the collaboration of professionals from 50 NICUs from 15 administrative regions in Spain (Figure 2). Eight workshops were held to train implementation teams on the management of the system and attended by a total of 210 health professionals (neonatologists and nurses).
The 45 units that ultimately started the local implementation process received a questionnaire asking about the details of the implementation, the perceived barriers and the strategies used to address them. The survey response rate was 71.1% (32 units). The strategies used to inform the staff were: 1) informative meetings for the entire NICU staff; 2) series of specific meetings for each type of professional on staff; and 3) meetings attended only by staff that expressed an interest in participating in the initiative. Twelve champion stated that in spite of their efforts to inform and motivate their colleagues, they ended up being the only staff directly involved in data collection and quality assurance. The problems reported most frequently by facilitators as having a negative impact on the implementation were: difficulty in establishing the NeoKissEs team due to lack of motivation among the staff, difficulty ensuring knowledge of the initiative by all NICU staff, a high turnover rate in the NICU, especially among nurses, and poor collaboration between nurses and physicians.
Several facilitators felt that the work of nurses in data collection for NeoKissEs would benefit from the support of professionals from outside the unit experienced in infection prevention and control, yet only two NICUs reported an ongoing involvement of preventive medicine colleagues in infection assessment and control in close cooperation with the nurse and neonatologist. This collaboration was greatly valued in both instances.
By the beginning of 2015, the NeoKissEs website was fully operational, allowing for direct data entry and hosting a collection of streaming video tutorials developed to resolve possible questions concerning the correct registration of cases and BSI episodes and to explain how to produce reports. The data recorded to that point were transferred to definitive electronic case report forms.
Real-time statistical reports could be obtained through the website to provide feedback to the champion. These reports offered: 1) general patient information, such as dates of admission, birth weights, etc, 2) statistics for their particular unit (rates of infection and device use) and 3) comparative analyses of their NICU and other units based on the summary statistics calculated by the system.
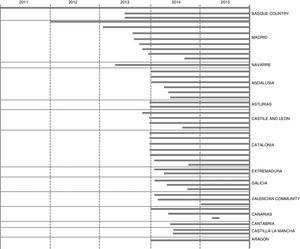
Participating NICUs gradually started to recruit patients, depending on the date that the facilitators attended the training and the time required to set up and launch the system in their hospitals (Figure 3). Of the 50 NICUs that initially joined the system, 45 had registered cases by December 31, 2015 (Figure 1). NeoKissEs continues to gather data at the time of this writing. All participating NICUs were classified as level III based on the level of care they offered: Level III-A (15 NICUs, 660 infants), level III-B (23 NICUs, 1863 infants) and level III-C (7 NICUs, 1115 infants).14
During the pilot phase, the two participating NICUs included 260 VLBW infants that were monitored for a total of 9324 days; of these infants, 81 (31.2%) developed CABSIs, with an overall incidence rate of 19.6 CABSIs per 1000 catheter-days (95% CI, 15.5-24.0). From the time that NICU recruitment started nationwide (February 8, 2013) through the end of 2015, 3750 infants were registered in the system, of who 112 (3.0%) were excluded from the analysis because they did not meet the inclusion criteria.
Table 1 summarises the perinatal characteristics of the 3638 infants that met the inclusion criteria. Overall, 966 VLBW infants (26.6%) had at least one episode of BSI during the followup in the system. Compared to VLBW infants without BSI, those with BSI had been born at lower gestational ages with lower birth weights and had a higher mortality during the surveillance period (relative risk, 1.8; 95% CI, 1.4-2.3). Furthermore, the device use rates (for vascular catheters and antibiotics) and length of stay recorded in the system were higher in this group (Table 1).
Perinatal characteristics, mortality and device use in patients that did and did not develop bloodstream infections (implementation phase).
| Parameter | All infants | No BSI | BSI | P |
|---|---|---|---|---|
| Patient characteristics | ||||
| Patients, n | 3638 | 2672 | 966 | |
| Total patient daysa | 131 485 | 85 420 | 46 065 | |
| Length of stay (days)aMedian (IQR) | 32.5 (23-46) | 29 (21-40) | 45 (31-63) | < .001d |
| Birth weight (g)Mean (SD) | 1116.6 (273.9) | 1176.2 (254.0) | 951.5 (259.0) | < .001d |
| < 1000g, n (%) | 1225 (33.7) | 664 (24.9) | 561 (58.1) | < .001e |
| Gestational age (weeks)Median (IQR) | 29 (27-31) | 30 (28-32) | 27 (26-29) | < .001d |
| < 28 weeks, n (%) | 1457 (40.0) | 832 (31.1) | 625 (64.7) | < .001e |
| CRIB scoreb, Median (IQR) | 1 (1-4) | 1 (0-2) | 2.5 (1-5) | < .001d |
| Sex (male), n (%) | 1823 (50.1) | 1313 (49.1) | 510 (52.8) | .051e |
| Delivery (caesarean), n (%) | 2697 (74.2) | 1994 (74.6) | 703 (72.8) | .260e |
| Multiple birth, n (%) | 1282 (35.2) | 959 (35.9) | 323 (33.4) | .171e |
| Mortality, n (%) | 274 (7.5) | 166 (6.2) | 108 (11.2) | < .001e |
| Rate of vascular catheter/antibiotic usec | ||||
| Vascular catheter,Median (IQR) | 40.0 (26.1-64.5) | 33.3 (22.9-51.4) | 64.1 (46.3-91.7) | < .001d |
| Antibiotic useMedian (IQR) | 21.1 (7.7-42.3) | 14.5 (0.0-28.5) | 44.2 (30.8-64.0) | < .001d |
Abbreviations: BSI, bloodstream infection; CRIB, Clinical Risk Index for Babies; IQR, interquartile range; SD, standard deviation.
a Until the end of the surveillance; b Score calculated in 2466 patients; c Number of catheter and/or antibiotic days per 100 patient days; d Mann-Whitney U test; e chi square test.
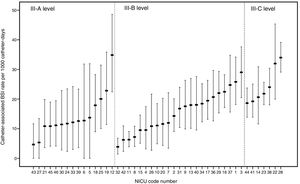
A total of 1108 episodes of CABSI were registered, corresponding to an incidence rate of 18.4 BSIs per 1000 catheter days (95% CI, 17.8-19.1). We found considerable variability in the CABSI rate between the 45 NICUs that registered cases during the study period (Figure 4). In the analysis by level of care, CABSI rates ranged from 4.7 to 34.9 in class III-A units, from 3.9 to 29.0 in class III-B units, and from 18.6 to 34.0 in class III-C units.
DiscussionOur results reflect the successful implementation in a substantial number of Spanish NICUs of the German system (NEO-KISS) validated for the surveillance of nosocomial BSIs in the VLBW infant population. Our study also produced initial data on standardised indicators (statistics on BSI, CABSI, and antibiotic and device use) and identify significant challenges and barriers that need to be addressed in the implementation and management of the surveillance system and of potential interventions for quality improvement.
Previous research has shown that the implementation of a BSI surveillance system specifically focused on VLBW infants may help reduce the impact of sepsis in NICUs.4 This positive effect may be due to the systematic collection of data through specific methods and the application of standardised definitions that facilitate the analysis and interpretation of statistics, the comparison between NICUs and feedback to clinicians.4,10,18
Our experience demonstrates that it is possible to adapt and implement a prospective surveillance system for BSI in the population of VLBW infants admitted to Spanish NICUs. With its standardised procedures and outcomes, NeoKissEs may well become the Spanish reference system for the collection and use of BSI data in VLBW infants for benchmarking and quality improvement purposes. The data from NeoKissEs supplements the information produced since 1994 by the Neonatal Network of the Castrillo Hospital Group in Spain,19 whose data collection is restricted to microbiologically-confirmed cases of neonatal BSI, but periodically provides useful reports on the antibiotic susceptibility of the isolated microorganisms. Our initial cumulative incidence (26.6%) was only slightly higher than the one described by the Castrillo Group (21.3%) (Fernández-Colomer B, Coto-Cotallo G.D. 27 October 2017, unpublished raw data) and in early reports of the German NEO-KISS system (21.3%).5 On the other hand, the BSI incidence rate per 1000 patient days and CABSI rate per 1000 catheter days found by NeoKissEs are much higher compared to the earliest benchmarking dataset obtained from the German system (9.2 vs 6.5 and 18.4 vs 9.2, respectively).5 This gap was even larger when we compared our data to the NEO-KISS values for the same time period (2013-2015) (3.8 BSI episodes per 1000 patient days and 6.5 CABSI episodes per 1000 catheter days) (Piening B, 24 November 2017, unpublished raw data), which highlights the role of NeoKissEs as a useful tool to stimulate research on potentially modifiable risk factors.
Our preliminary findings also brought to light other issues that need considering. First of all, we found considerable heterogeneity in the approaches used to implement the surveillance system at each site, despite the general consensus achieved in the implementation workshops. Some NICUs reported difficulties forming multidisciplinary teams to direct the implementation process, ending with a single person being responsible for all data collection activities and the impeding independent data validation at the NICU level. Further, accurate measurement and reporting is of critical importance but of limited effect if not accompanied by organised efforts to address the main factors associated with an increased risk of BSI. The reported difficulties in maintaining stable implementation teams could be a major barrier to these units benefitting from benchmarking information to drive the design and implementation of better practices.20
Secondly, the responses to the survey showed that there was substantial variability in the resources available in the NICUs, their organisational characteristics, their caseloads, staffing, levels of motivation, reward systems and quality assurance policies. All these factors should be taken into account in the near future, when, as we hope, NeoKissEs becomes the national framework for the introduction and evaluation of evidence-based best practices and quality improvement initiatives focused on the prevention and control of BSI in VLBW infants.
Thirdly, the huge variability observed so far in the frequency of BSI episodes, even in NICUs with the same level of care (Figure 4), suggests the existence of potentially modifiable health care-related factors. This calls for in-depth examination of these factors and the development of an appropriate framework for targeted actions aimed at reducing the rates of preventable infection in the future. Many NeoKissEs units are currently participating in a new interventional study to test the systematic implementation of a set of bundles of measures concerning the insertion and maintenance of central vascular catheters in VLBW infants with the aim of reducing CABSI rates. NeoKissEs will continue to support the collection of BSI statistics for these units and will be used to assess the impact of the bundle interventions.
The creation of a web-based electronic support system has greatly facilitated the consecutive registration of patients and BSI episodes and the monitoring of data quality. It allows NICUs to consult their statistics, for their own unit or in comparison with others, at any time. It also helps identify areas for improvement by comparing their results with the overall data provided by NeoKissEs and the data from the German NEO-KISS. Comparisons may be biased if there is heterogeneity in the compared populations (case mix bias).21 To address this, the system carries out adjusted analyses, providing comparisons that are more valid by taking into account factors such as birth weight, gestational age and the complexity of the provided care.
The number of NICUs that participate in NeoKissEs ensures a sufficiently large sample of individuals and BSI episodes to provide accurate and reliable statistics and facilitate the performance of studies of appropriate scope to evaluate the safety and effectiveness of specific interventions and policies. That said, expanding the NeoKissEs system would make the data more representative of the whole of Spain. There is the limitation that NeoKissEs was developed in the framework of a research project funded for a limited period of time and that it has yet to be permanently integrated into the Spanish National Health System, unlike NEO-KISS, in which participation has been mandatory for all German neonatal units since 2006. It would also be advisable for NeoKissEs to establish collaborations with other similar initiatives, registries and neonatal networks to share data, expand benchmarking activities and encourage the systematic analysis of the risks associated with the development of BSI in this population.
ConclusionsNeoKissEs has been implemented in 45 Spanish NICUs and has proven to be a useful source of information on the incidence of BSI and important associated factors. This standardised system is easy to use and widely accepted by users, and provides standardised definitions, procedures and data collection instruments that allow comparative assessments. We believe that this system would be a good tool for evaluating ongoing interventions aimed at improving health care quality and to promote the implementation of safe practices in participating NICUs, individually or as a group. It would no doubt benefit from being used by a larger number of NICUs, greater integration in the daily activity of health care staff and sustained institutional support.
StatementsEthics approval and consent to participateThe NeoKissEs protocol was first assessed and approved by the Basque Country Clinical Research Ethics Committee (PI2013151). Subsequently, the protocol was further evaluated and approved by the regional and local ethics committees overseeing each participating hospital. The requirement of written individual informed consent was waived due to the nature of the research and the use of anonymised data.
FundingThis project was funded by the Carlos III Research Institute through grant PI13/00587 and by the Red SAMID through grant RD12/0026 (both co-funded by the European Regional Development Fund/European Social Fund).
AuthorshipMM was the main author responsible for writing this article and its critical edition, and also contributed to the implementation of the surveillance system.
MCL was the neonatologist responsible for the NeoKissEs surveillance system and its implementation. She collaborated in drafting the manuscript.
JP collaborated in writing the manuscript, producing tables and graphs and organising the final version.
JE worked on the development and analysis of databases.
ES collaborated in the implementation of the surveillance system, and helped write the methods section of the manuscript.
BP made substantial contributions to the adaptation and interpretation of the definitions and instruments of the original German NEO-KISS. He was involved in the comparative analysis of NeoKissEs indicators with the German NEO-KISS reference population.
JIV was the principal investigator of the NeoKissEs system (PI13/00587) and led the implementation of the surveillance system. He collaborated in the writing of the manuscript.
JIP was responsible for analysing and interpreting the data obtained through the NeoKissEs system. He designed the methodology of the study and coordinated the writing of the manuscript.
All authors participated in the critical review of the manuscript and approved the final version.
The professionals of the NeoKissEs Group collected patient data in their respective hospitals for the surveillance system. They also participated in the completion of surveys and contributed to the accuracy of the final data by addressing the concerns brought up by the coordination team.
We thank all the staff and clinicians of the neonatal intensive care units that participated in the NeoKissEs-PI13/00587 nosocomial infections research project for their support and contributions. We thank Petra Gastmeier, Christine Geffers, Michael Behnke, Luis-Alberto Peña Díaz and Frank Schwab from the German National Reference Center for Surveillance of Nosocomial Infections for their methodological and technical support in this project. We also thank Casilda Arranz Cerezo for her help in designing the case report form and the editors of Ideas Need Communicating Language Services S. Coop for their help in improving the use of English in the manuscript. Lastly, we wish to posthumously acknowledge Adolfo Valls-i-Soler, who conceived the original idea underlying this work.
Teresa Jesús Agra Laya, Almudena Alonso Ojembarrena, Israel Anquela Sanz, Yolanda Armendáriz Cuevas, Cristina Barcelona Alfonso, José Beceiro Mosquera, María Bengoa Caamaño, Elena Bergón Sendín, Lucía Cabanillas Vilaplana, Fernando Cabañas González, Eva Capdevila Cogul, Javier Casanovas Lax, María Cernada Badía, Gil Daniel Coto Cotallo, Pilar Adelaida Crespo Suárez, María Isabel de las Cuevas Terán, Laura Domingo Comeche, Izaskun Dorronsoro Martín, Pilar Espiño Lorenzo, Marta Estalella Bellart, Francisco Javier Estañ Capell, Belén Fernández Colomer, José Luis Fernández Trisac, Zenaida Galve Pradel, Miguel Ángel García Cabezas, María García Franco, María Jesús García García, Victoria Eugenia García Rodríguez, Rafael García Mozo, Rubén García Sánchez, Fermín García-Muñoz Rodrigo, Silvia Garrido Esteban, Carmen González Armengod, Paloma González Carretero, María González López, María Mercedes Granero Asencio, José María Hernández Hernández, María Elena Infante López, Ana Irasarri Sebastián, Francisco J Jiménez Parrilla, Pedro J Jiménez Parrilla, María Isabel Larburu Aristizabal, Manuela López Azorín, Juan María López de Heredia Goya, Jesús Cecilio López-Menchero Oliva, Salud Luna Lagares, Carmen Luz Marrero Pérez, Emilia María Martínez Tallo, Andrés Martínez Gutiérrez, María Dolores Martínez Jiménez, María de los Ángeles Martínez Fernández, Raquel Mendiola Ruiz; María Leticia Millán Miralles, Alicia Mirada Vives, Jesús Molina Cabrillana, Elisenda Moliner Calderón, Icíar Olabarrieta Arnal, Antonio Pavón Delgado, Alberto Pérez Legorburu, Alejandro Pérez Muñuzuri, Raquel Pinillos Pisón, Segundo Rite Gracia, Sonia M Rivero Rodríguez, Silvia Rodríguez Blanco, Gerardo Romera Modamio, María Dolors Salvia Roigés, Mario Sánchez Fernández, Antonio Segado Arenas, Eduard Solé Mir, Itziar Sota Busselo, Joaquín Suárez Fernández, José Luis Tarazona Fargueta, Cinzia Tripodi, María Purificación Ventura Faci and Javier Vilas González.
Please cite this article as: Madrid-Aguilar M, López-Herrera MC, Pérez-López J, Escudero-Argaluza J, Santesteban-Otazu E, Piening B, et al. Implementation of NeoKissEs in Spain: A validated surveillance system for nosocomial sepsis in very low birth weight infants. An Pediatr (Barc). 2019;91:3–12.