Despite being a rare disease, cancer is the first cause of mortality due to disease during the paediatric age in the developed countries. The current, great increase in new treatments, such as immunotherapy, constitutes a new clinical and regulatory paradigm. Cellular immunotherapy is one of these types of immunotherapy. In particular, the advanced therapy drugs with chimeric antigen receptors in the T-lymphocytes (CAR-T), and particularly the CAR-T19 cells, has opened up a new scenario in the approach to haematology tumours like acute lymphoblastic leukaemia and the B-Cell lymphomas. The approval of tisagenlecleucel and axicabtagene ciloleucel by the regulatory authorities has led to the setting up the National Plan for Advanced Therapies-CAR-T drugs in Spain. There is evidence of, not only the advantage of identifying the centres most suitable for their administration, but also the need for these to undergo a profound change in order that their healthcare activity is extended, in some cases, to the ability for the in-house manufacture of these types of therapies. The hospitals specialised in paediatric haematology-oncology thus have the challenge of progressing towards a healthcare model that integrates cellular immunotherapy, having the appropriate capacity to manage all aspects relative to their use, manufacture, and administration of these new treatments.
A pesar de ser una enfermedad rara, el cáncer es la primera causa de mortalidad por enfermedad durante la edad pediátrica en los países desarrollados. En este momento, la irrupción de nuevos tratamientos como la inmunoterapia constituye un nuevo paradigma clínico y regulatorio. Uno de estos tipos de inmunoterapia es la inmunoterapia celular. En particular, los medicamentos de terapia avanzada con receptores antigénicos quiméricos en los linfocitos T (CAR-T), y en concreto las células CAR-T19, han supuesto un nuevo escenario en el abordaje de los tumores hematológicos, como la leucemia aguda linfoblástica y los linfomas de células tipo B. La aprobación por las autoridades regulatorias de tisagenlecleucel y axicabtagene ciloleucel, ha impulsado la puesta en marcha del Plan Nacional de Terapias Avanzadas-Medicamentos CAR-T en España, evidenciándose no sólo la conveniencia de identificar los centros más adecuados para su administración, sino la necesidad de que éstos sufran una profunda transformación para que su actividad asistencial se extienda en algunos casos a la capacidad de fabricación propia de este tipo de terapias. Los hospitales especializados en hemato-oncología pediátrica tienen por tanto el reto de evolucionar hacia un modelo asistencial que integre la inmunoterapia celular, dotándose de capacidad propia para gestionar todos los aspectos relativos al uso, fabricación y administración de estos nuevos tratamientos.
Until the second half of the past century, leukaemia, the most frequent cancer in the paediatric age group, was an incurable disease. In 1948, Sidney Farber was the first researcher to demonstrate that a drug that inhibited the folate pathway, aminopterin, could achieve temporary remissions of leukaemia.1 Later, Donald Pinkel introduced the concept of “total therapy”, an approach in which the combination of chemotherapy agents synergistically induced prolonged remissions.1 Successive optimisations of this initial strategy have succeeded in turning childhood acute leukaemia a curable disease in most cases.2–4 However, even in developed countries today, cancer continues to be the leading disease-related cause of death in children and adolescents.
The development and impact of cancer treatments in the past decade is the fruit of decades of research.5–11 Unlike conventional treatments, immunotherapy aims to utilise, manipulate and augment the defences of the organism to attack and eliminate tumour cells. At present, cancer immunotherapy is the largest field in ongoing clinical trials worldwide. The term “cancer immunotherapy” encompasses a broad range of therapeutic options, including monoclonal antibodies, immune checkpoint inhibitors, vaccines, oncolytic viruses, cytokines, adjuvant immunotherapy and adoptive immunotherapy with T cell and natural killer (NK) cell transfers (Table 1). However, the recent and renewed momentum of immunotherapy and the greatest clinical and social interest it is currently attracting revolves around adoptive immunotherapy strategies based on the genetic modification of lymphocyte T cell receptors (TCRs) and the development of chimeric antigen receptors (CARs), with more than 700 clinical trials underway worldwide investigating their use for treatment of blood cancers and, to a lesser extent, solid tumours.
Types of cancer immunotherapy.
| Types of cancer immunotherapy | ||||||
|---|---|---|---|---|---|---|
| Active immunotherapy (amplification of immune response) | Passive immunotherapy (initiation of a new immune response) | |||||
| Immune checkpoint inhibitors | Immune checkpoint agonists | Anti-tumour vaccines/innate immune response activators | Monoclonal antibodies (naked, conjugated and bispecific) | Oncolytic viruses | Adoptive T cell therapy | |
| Objective | Increase susceptibility of tumour cells to attack by immune system | Stimulate immune system to attack tumour | Specifically target tumour cells | Direct destruction of tumour cells while triggering a significant immune response | Enhance natural activity of T-cells against cancer | |
| Mechanism of action | Block mechanisms that inhibit the immune response (CTLA-4, PD- 1/PDL-1) | Stimulate antigen-presenting cells and T-cells | Modulate immune response (monocytes/macrophages) | Localization of cell-surface antigens they have been designed to recognise:Activation of various immune mechanismsLead the drug to attack tumour cells | Direct lysis of tumour cells and activation of immune cells | Enhances natural anti-tumour activity of T-cells through their ex vivo modification and subsequent infusion to patient. |
| Name and indications therapy is approved for | CTLA-4 inhibitorsIpilimumab:melanoma in patients >12 years and adults (EMA and FDA)PD-1/PD-L1 inhibitorsNivolumab: colorectal cancer in patients >12 years (FDA) and melanoma, lung cancer, kidney cancer, Hodgkin lymphoma, lymphoma of head or neck, of the bladder in adults, adults (EMA and FDA)Pembrolizumab:Hodgkin lymphomaR/R primary mediastinal large B cell lymphoma, high-MSI or non-CNS or MMRD tumours in paediatric patientsFor all indications in paediatric and adult patients (FDA) and only in adults (EMA)Avelumab: Mediastinal Merkel cell carcinoma in paediatric patients >12 years and adults (FDA) and only in adults (EMA) | Mifamurtide: paediatric osteosarcoma (EMA) | Dinutuximab beta: neuroblastoma in paediatric patients >12 months (EMA)Blinatumomab: paediatric and adult B-cell ALL (EMA and FDA)Gemtuzumab ozogamicin: AML in patients >15 years (EMA) and paediatric or adult patients (FDA) | Tisagenlecleucel: post-transplant B-cell ALL that is refractory or in second or third relapse in paediatric patients or young adults <25 years (EMA and FDA). R/R DLBCL after 2 or more lines of systemic treatment adults (EMA and FDA) | ||
| Clinical trials or under study (most relevant) | PD-1/PD-L1 inhibitors Other indications in the paediatric population: melanoma, glioblastoma with MMRD, several rare tumours, anaplastic Alk + lymphoma, CNS lymphoproliferative disorder | Initial stages of investigation | Rituximab: autoimmune diseases in paediatric populationHu3F8: neuroblastoma in paediatric populationHu14.18K322A: neuroblastomain paediatric population | Reolysin:osteosarcoma and soft tissue sarcomas: Ewing sarcoma, malignant fibrous histiocytoma, fibrosarcoma, leiomyosarcoma (in paediatric and adult population)HSV G207: paediatric brain tumours | CD22 CAR T, CD19 CAR T, dual CD19/22 CAR T: leukaemia in paediatric populationCD22 CAR T: lymphoma in paediatric populationHER2 CAR T, EGFR CAR T: R/R CNS tumoursGenetically modified TIL: metastatic melanoma in paediatric populationAENK cells:Solid tumours (sarcomas) and blood tumours (leukaemia, lymphoma) in paediatric patients | |
AENK, activated and expanded natural killer cells; ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; CAR, chimeric antigen receptor; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; EMA, European Medicines Agency; FDA, Food and Drug Administration; MMRD, mismatch repair deficiency; MSI, microsatellite instability; R/R, refractory/recurrent; TIL, tumour-infiltrating lymphocytes.
Paediatric haematology and oncology departments and units in Spain are exhibiting a clear interest in immunotherapy.12,13 This interest is evinced in the efforts made in the past and present by many institutions in forming multidisciplinary teams and adapting or creating the necessary infrastructures to implement these pioneering therapeutic programmes (either as treatment facilities or simultaneously as development and treatment facilities), or with active participation in preclinical development and clinical trials of these novel therapies. A consultation made in 2018 within the Group on Immunotherapy and Advanced Therapies of the la Sociedad Española de Hematología y Oncología Pediátricas (Spanish Society of Paediatric Haematology and Oncology) found that as many as 13 sites had an interest in CAR-T cells, NK cells, tumour-infiltrating lymphocytes, oncolytic viruses and monoclonal antibodies for treatment of both solid tumours (sarcomas and central nervous system tumours) and mainly of leukaemias. Furthermore, 9 facilities reported having their own infrastructure or access to authorised facilities for administration of cellular therapies and the manufacture of advanced targeted therapies (ATTs), while 4 facilities were accredited by the Joint Accreditation Committee International Society for Cellular Therapy Europe & European Group for Blood and Marrow Transplantation (JACIE) and another 4 were in the process of pursueing this accreditation.
CAR-T cell therapyChimeric antigen receptor (CAR) T cell therapy emerged from the combination of advances in cancer humoral-targeted immunotherapies14 and the genetic manipulation of T cells (CAR-T cells).15,16 A CAR-T cell has antigen-recognition domains obtained from the variable regions of the heavy and light chains of a specific monoclonal antibody joined to form a single-chain Fv (scFv) domain. This scFv domain is fused to an intracellular CD3ζ TCR signalling motif. In the early days, Dr Zelig Eshhar called this strategy “T-body”.17 The first CAR T cells developed through this approach are known as first-generation CAR T cells.
At present, this technique has evolved to incorporate signalling motifs from different domains from costimulatory molecules such as CD28, OX40 or 41BB, giving rise to second- and third-generation CAR T cells. As an alternative to scFV, it is also possible to use domains from receptors such as NKG2D, CD16 or Erb-B.18,19 In theory it is possible to design CAR T cells to target any extracellular antigen, but those that target antigen CD19 (CART-19), which is expressed in B-cell lymphomas and B-cell acute lymphoblastic leukaemia (ALL) have been most clinically successful to date, and 2 commercial autologous T cell brands, Kymriah® (tisagenlecleucel, manufactured by Novartis) and Yescarta® (axicabtagene ciloleucel, manufactured by Gilead/Kite Pharma) started to be marketed in the United States in autumn of 2017 and in the European Union in summer of 2018.20–22
In Spain, academic institutions are developing CAR T-19 therapies parallel to the development in industry. The first public hospitals that promoted this strategy were the Hospital Clínic and the Hospital Sant Joan de Déu in Barcelona for treatment of paediatric patients with B-cell ALL and adults with B CD19+ lymphoma.23 At present, clinical trials of CAR T cells in Spain are underway in both industry and academic settings.
From a regulatory standpoint and for the purpose of development and manufacture, CAR T cells are considered advanced targeted therapies. The development and trial of medicines comprehends several phases aimed at guaranteeing the fundamental quality, safety and efficacy requirements expected of any drug.24–27 Preclinical in vitro and in vivo studies are the proof-of-concept phase that precedes performance of clinical trials. Confirmation of efficacy in phase III trials allows application for marketing authorization in the European Union. Until a few years ago, advanced targeted therapies had limited commercial success, but at present the industry has taken on this challenge and is developing these treatments. Nevertheless, academia continues to be the main drive of technological advancement in this type of therapies, which is reflected in the growing number of collaborations between academic institutions and industry partners.
CAR-T cell therapy in the hospital settingCAR-T cell immunotherapy is associated with significant morbidity, especially in patients with a heavy tumour burden. The management of potential complications, such as cytokine release syndrome, neurologic changes, prolonged cytopenias or B-cell aplasia, requires multidisciplinary teams with experience in these complications, some of which are specific to CAR-T cell therapy and others commonly found in the context of allogeneic stem cell transplantation.
The delivery of treatments based on CAR-T cell immunotherapy requires a multidisciplinary team of different health professionals to carry out each phase of the process from administration to the patient through post-administration care: investigation of the clinical case and admission to the treatment protocol, collection of source material, submission of material to the external manufacturer or in-house manufacturing facility, patient conditioning with lymphodepleting chemotherapy, CAR-T cell infusion, follow-up and treatment of early and late post-infusion complications and legal and regulatory aspects.28–30 Therefore, the team responsible for the process must include different specialists and be well coordinated: haematologists, oncologists, intensive care physicians, paediatricians, neurologists, immunologists, biologists, geneticists, pharmacists, hospital pharmacists, regulation experts, nurses, professionals in different supportive care specialities and experts in different quality assurance systems. Since November 2018, the National Health System of Spain (Sistema Nacional de Salud, SNS) has established a National Plan for the delivery of ATTs and the administration of CAR-T cells, with establishment of regional plans by each regional government. The aim of the plan was to structure the delivery of these therapies in a planned, equitable, safe and efficient manner under conditions guaranteeing quality, safety and efficacy standards. The plan was developed in collaboration with the governments of each autonomous community of Spain, scientific societies and patient associations, and included a strategic plan to promote public research and for the translation of research to clinical practice and the in-house manufacturing of CAR-T cells by academic institutions. On March 4, 2019, the ministry established criteria and standards for the designation of centres authorised to use CAR-T cell therapies in the public health system.
In April 2019, the Spanish Ministry of Health published the list of the Spanish centres authorised for delivery of CAR- T cell therapy. It designated 8 centres for treatment of adult patients with refractory or recurrent diffuse large B-cell lymphoma (DLBCL) or refractory B-cell ALL. In the case of paediatric patients, it has designated 3 centres for treatment of refractory B-cell ALL. In addition, it designated 3 centres for delivery of CAR-T cell therapy in case capacity is exceeded in the other centres: 2 for adults and 1 for paediatric patients.
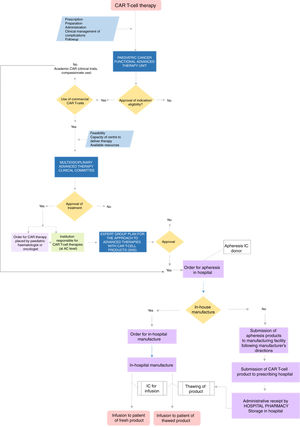
Centres authorised to deliver CAR-T cell therapy must have multidisciplinary units to address every aspect involved in the use of this treatment and the clinical management of the patients. The team must include the professional in charge of the CAR-T cell programme, clinicians involved in care delivery and other professionals. Fig. 1 presents a flow chart of the care delivery process for CAR-T cell therapy.
Since CAR-T cells are considered an ATT, the regulatory standards for their manufacture directly affect the infrastructure and staff that are directly or indirectly involved in their production.31,32 This is a particularly significant challenge in the research hospital setting, as good manufacturing practice (GMP) facilities, traditionally known as clean or white rooms, are required.19,33,34 Historically, GMPs have been applied to the manufacture of conventional drugs and medicinal products, so their application to the production of ATTs and the manipulation of cells and tissues requires considerable adaptation.35 Furthermore, the management of a pharmaceutical manufacturing facility for production of medicinal products in a hospital setting poses multiple practical challenges, to the extent that recently, the Committee for Advanced Therapies of the European Medicines Agency (EMA) established specific regulations for these medicinal products.36 Thus, the manufacture in hospitals of advanced therapies in general and of CAR-T cell products in particular requires the integration of professionals specialised in different fields and therefore modifications to the care delivery model.
Delivery of CAR-T cell therapyOnce the prescription is made and authorised, the patient is scheduled for lymphapheresis. The autologous treatments currently available, tisagenlecleucel, axicabtagene ciloleucel and academic CD19 CAR-T cell (CART-19) therapy require a minimum number of T cells capable of expansion, and therefore it is critically important to identify the optimal timing for cell collection in order to guarantee the quality and quantity of this white blood cell population. The apheresis product must be cryopreserved for manufacture of tisagenlecleucel, but this is not necessary for axicabtagene ciloleucel. In the case of the 2 brands that are manufactured externally, the source material collected at the hospital is submitted to the CAR-T cell manufacturing facility, and once the product has been manufactured, it is sent back to the prescribing hospital. The entire supply chain must meet the applicable legislation at each stage of the manufacturing process to guarantee the quality, safety and traceability of the product, which requires staff trained in apheresis, cellular therapies and nurse care coordinators to organise patient care and related logistics. Hospital pharmacy departments play an essential role in the chain of custody from the order to the receipt of the manufactured medicinal product, as well as in subsequent pharmacovigilance. The average turnaround time in the manufacture of commercial CAR-T cells from cell collection, through submission to the manufacturing facility and to the reception of the manufactured product is variable and estimated to range from 17 to 60 days. In the days preceding CAR-T cell infusion, the patient prepares for the procedure, and clinicians assess the clinical condition of the patient and the course of disease. The patient then undergoes a lymphodepletion protocol to facilitate CAR-T cell expansion after the infusion. During infusion and in the post-infusion period, it is essential that the patient be monitored for potential adverse events (neurotoxicity, cytokine release syndrome etc.), so, of the different medical specialities that need to be involved in the multidisciplinary teams, active participation of paediatric neurology and paediatric intensive care specialists is particularly important. Several scientific societies, such as the American Society of Gene and Cell Therapy, the Foundation for the Accreditation of Cell Therapy and the International Society for Cellular Therapy, are developing guidelines to standardise the delivery of CAR-T cell therapies and for the management of their potential adverse effects.
Social and economic impact of CD19 CAR-T cell therapyIn December 2018, the Spanish Ministry of Health, Consumption and Social Welfare announced the funding for the first CAR-T cell therapy (CART-19, tisagenlecleucel) to be used in the public health system for treatment of refractory B-cell ALL and DLBCL, and the first case of a paediatric patient with B-cell ALL cured with this therapy was recently reported by the Hospital Sant Joan de Déu. In July 2019, the Ministry approved the price and funding of the second commercial CAR- T cell therapy (CART-19, axicabtagene ciloleucel) for treatment of adults with 2 types of non-Hodgkin lymphoma: DLBCL and primary mediastinal large B-cell lymphoma. In Spain, the funding of both of these treatments (tisagenlecleucel and axicabtagene ciloleucel) is subject to two-sided risk payment agreement. We ought to underscore that to the cost of the product we have to add the costs of hospitalization, supportive care and specific drugs for complications and interleukin-6 inhibitors.37
Limitations and future of CAR-T cell therapiesCAR-T cell therapies have been quickly introduced to the clinical setting due to the need to treat patients with refractory disease or multiple recurrences. In the specific case of B-cell ALL, the response rates are historically greater compared to the rates observed in chronic lymphocytic leukaemia and high-grade lymphomas. The reasons for this differential response, in the case of CART-19 in B-cell ALL, lymphoma and chronic lymphocytic leukaemia, may be multiple and intrinsic to the tumour cell. However, a growing body of evidence suggests that the quality of the autologous T cells used as effector cells to be CAR-transduced has a substantial impact on treatment response. Unfortunately, the “functionality” of T cells has been frequently assessed in clinical samples obtained at the time of diagnosis, whereas few studies have prospectively assessed the quality of T cells in recurrences or in more advanced stages of disease. Therefore, studies are needed that include functional assessments of T cells in stages of disease close to the potential inclusion of a patient in a CAR-T cell trial. These studies should be longitudinal and compare the characteristics and function of T cells at diagnosis with those of healthy donors matched for age, and the differences in paired samples of T cells collected at diagnosis and at the time of recurrence. These studies should collect data on: a) the number of T cells available for use in adoptive immunotherapy; b) the distribution of lymphocyte populations with particular emphasis on γδ and memory T cells; c) the proliferative, activation and proinflammatory capacity in response to antigen exposure, and d) the expression of immune checkpoints, which tend to increase significantly in advanced stages of disease, thus facilitating immune evasion and T cell depletion.38,39 The manufacture of universal donor-derived allogeneic CAR-T cells and their availability as cryopreserved therapies is a challenge for the upcoming future. To achieve this objective, we need to develop safer gene editing systems to both guarantee effective transduction and allow the use of T cells in a human leukocyte antigen (HLA)–independent manner.
On the other hand, the main limitation of CAR-T cell therapy at present, in addition to the complications of treatment, is its cost and the complexity of its manufacture. The possibility of using CAR-T cells produced in academia is both a great challenge and a great opportunity. Lastly, therapies from other effector cell sources, such as NK cells (CAR-NK) derived from umbilical cord blood or the memory T cell populations, must be developed to overcome all the current limitations of CAR-T cell therapy.
ConclusionWe are experiencing a revolutionary time in the management of paediatric cancer patients as immunotherapy is added to conventional treatments with extraordinary impact. To date, CAR T-19 therapy has been the most successful immunotherapy modality and has already been approved for use in children and young adults up to age 25 years with refractory or second relapse B-cell ALL. For the time being, 2 commercial brands have received approval for CAR-T cell therapy, although only one has been authorised for the paediatric age group, and a logistics model must be established in accordance with pharmaceutical standards for their global manufacture and distribution to a large number of patients whose adequacy will be tested with experience. On the other hand, anything concerning bridging therapy prior to CAR-T cell administration and the subsequent management of complications will correspond to another learning curve, so each facility will also have to establish its own standards regarding the use of these products. The in-house manufacturing model, with production within each facility in a closed model, and the simplification of the construction of strictly controlled facilities in adherence with the quality and safety standards that apply to any facilities that manufacture medicinal products will make it possible to, on one hand, optimise processes and their costs while increasing patient safety and ensuring excellence of care and competitive care delivery as strategies are disseminated with this decentralization, and on the other to promote teaching and research in the field of paediatric haematology and oncology.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the CRIS Cancer Foundation.
Isabel Mirones. Translational Research and Advanced Targeted Therapies Unit, Department of Paediatric Haematology and Oncology, Hospital Universitario La Paz, Madrid.
Luisa Sisinni. Department of Paediatric Haematology and Oncology, Hospital Universitario La Paz, Madrid.
Marina García-Morín. Department of Paediatrics, Hospital General Universitario Gregorio Marañón, Madrid.
Javier Anguita. Department of Haematology, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón (IiSGM), Madrid.
Manuel Ramírez, Miguel Ángel Díaz and Marta González. Advanced Therapies Unit, Department of Paediatric Haematology and Oncology, Hospital Universitario Niño Jesús, Madrid.
Lucas Moreno and Laura Alonso. Department of Paediatric Haematology and Oncology, Hospital Universitario Valld'Hebron, Barcelona.
Susana Rives. Department of Paediatric Haematology and Oncology, Hospital Sant Joan de Déu, Barcelona.
Marta M. Alonso and Ana Patiño-García. Instituto de Investigación Sanitaria de Navarra (IDISNA), Pamplona, Spain; Solid Tumours and Biomarkers Programme, Fundación para la Investigación Médica Aplicada, Pamplona, Spain; Department of Paediatrics, Clínica Universidad de Navarra, Pamplona, Spain.
Pilar Palomo. Haematopoietic Stem Cell Transplantation Unit, Department of Haematology and Haemotherapy, Hospital Central de Asturias, Oviedo.
Jaime Verdú-Amorós. Paediatric Haematology and Oncology Unit, Hospital Clínico Universitario, Valencia.
Isabel Martínez. Paediatric Haematology and Oncology Unit, HM Hospitales, Madrid.
Garbiñe Lizeaga. Department of Pharmacy, Hospital Universitario Donostia, San Sebastian, Gipuzkoa, Spain.
Pilar Guerra-García. Paediatric Haematology and Oncology Unit, Hospital Universitario 12 de Octubre, Madrid.
José Luis Fuster, José M. Moraleda, Andrés Sánchez-Salinas and Miguel Blanquer. Area of Paediatric Haematology and Oncology, Haematopoietic Stem Cell Transplantation and Cell Therapy Unit, Department of Haematology, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain; Instituto Murciano de Investigación Biosanitaria (IMIB), Universidad de Murcia, Murcia, Spain.
Javier García-Castro. Cellular Biotechnology Unit, Instituto de Salud Carlos III, Majadahonda, Madrid.
María Luisa Toribio e Hisse M. van Santen. Environmental Interaction Programme, Immune System Development and Function Unit, Centro de Biología Molecular Severo Ochoa, CSIC-UAM, Madrid.
Pablo Menéndez. Instituto de Investigación contra la leucemia Josep Carreras, School of Medicine, Universidad de Barcelona, Barcelona; Instituto Catalán de Recerca i Estudis Avancats (ICREA) Research Professor; Centro de investigación en Res de Cancer (CIBERONC), ISCIII, Barcelona.
Antonio Pérez-Martínez. Translational Research on Paediatric Haematology and Oncology Unit, Haematopoietic Stem Cell Transplantation and Cell Therapy Unit, Hospital Universitario La Paz, Madrid, Spain; Department of Paediatric Haematology and Oncology, Hospital Universitario La Paz, Madrid, Spain.
Appendix A includes more detailed information on the Group on Immunotherapy and Advanced Therapies of the Sociedad Española de Hematología y Oncología Pediátricas.
Please cite this article as: Mirones I, Moreno L, Patiño-García A, Lizeaga G, Moraleda JM, Toribio ML, et al. Inmunoterapia con células CAR-T en hematooncología pediátrica. An Pediatr (Barc). 2020;93:59.