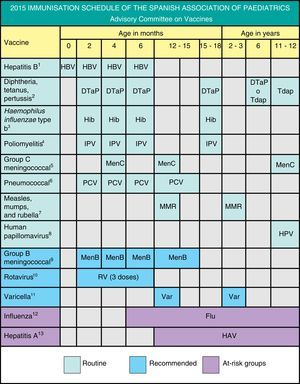
The Advisory Committee on Vaccines of the Spanish Association of Paediatrics updates the immunisation schedule every year, taking into account epidemiological data as well as evidence on the safety, effectiveness and efficiency of current vaccines, including levels of recommendation. In our opinion, this is the optimal vaccination calendar for all children resident in Spain.
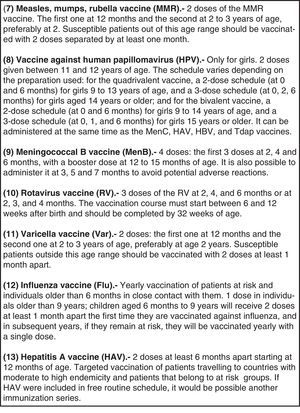
Regarding the vaccines included in the official unified immunization schedule, the Committee emphasizes the administration of the first dose of hepatitis B either at birth or at 2 months of life; recommends the first dose of MMR and varicella vaccine at the age of 12 months, with the second dose at the age of 2–3 years; DTaP or Tdap vaccine at the age of 6 years, followed by another Tdap booster dose at 11–12 years; Tdap strategies for pregnant women and household contacts of the newborn, and immunization against human papillomavirus in girls aged 11–12 years with a 2-dose scheme (0, 6 months).
The Committee reasserts its recommendation to include vaccination against pneumococcal disease in the routine immunisation schedule, the same as that conducted in Western European countries.
The recently authorised meningococcal B vaccine, currently blocked in Spain, exhibits the profile of a universal vaccine. The Committee insists on the need to have the vaccine available in community pharmacies.
It has also proposed the free availability of varicella vaccines. Their effectiveness and safety have been confirmed when they are administered from the second year of life. Vaccination against rotavirus is recommended in all infants. The Committee stresses the need to vaccinate population groups considered at risk against influenza and hepatitis A.
El Comité Asesor de Vacunas de la Asociación Española de Pediatría actualiza anualmente su calendario de vacunaciones, tras un análisis tanto epidemiológico como de la seguridad, efectividad y eficiencia de las vacunas actuales, incluyendo grados de recomendación. Es el calendario que se estima idóneo actualmente para los niños residentes en España.
En cuanto a las vacunas oficiales incluidas en el calendario común, se recalca la posibilidad de vacunar indistintamente frente a hepatitis B desde el nacimiento o desde los 2 meses; la recomendación de la primera dosis de triple vírica y de varicela a los 12 meses y la segunda a los 2-3 años; la administración de la vacuna DTPa o Tdpa a los 6 años, con refuerzo en la adolescencia; estrategias con Tdpa en embarazadas y convivientes del recién nacido, y la inmunización frente al papilomavirus en niñas a los 11-12 años con pauta de 2 dosis (0, 6 meses).
Este comité insiste en la vacunación antineumocócica universal, tal y como se está llevando a cabo en todos los países de Europa Occidental.
La vacuna frente al meningococo B, autorizada pero bloqueada actualmente en España, presenta un perfil de vacuna sistemática y se reivindica que, al menos, esté disponible en las farmacias comunitarias.
Se propone, igualmente, la disponibilidad pública de las vacunas frente a la varicela, ya que han demostrado ser efectivas y seguras a partir del segundo año de vida. La vacunación frente al rotavirus es recomendable en todos los lactantes. La vacunación antigripal anual y la inmunización frente a la hepatitis A están indicadas en grupos de riesgo.
The Advisory Committee on Vaccines of the Spanish Association of Paediatrics (CAV-AEP) updates the immunisation schedule every year, taking into account current evidence to propose the vaccine schedule that it considers most appropriate for children residing in Spain.
We present different grades of recommendation for the different vaccines in order to establish priority levels. They are explained in Fig. 1, which shows the schedule proposed by this Committee for 2015.
The Ministry of Health, Social Services, and Equality (Ministerio de Sanidad, Servicios Sociales e Igualdad [MSSSI]) has proposed a minimal nationwide routine immunisation schedule that in many regards is based on economic criteria rather than the scientific literature or the recommendations of its own health experts. Health professionals disagree with this schedule, and its implementation is facing problems in every autonomous community (AC). It does not adhere to the current recommendations of many official institutions and scientific associations (SAs), including the AEP, to vaccinate children younger than 5 years against pneumococcus or young children against varicella; to vaccine against human papillomavirus at 11–12 years of age; to allow vaccination against hepatitis B starting either at birth or at 2 months of age; or to vaccinate adolescents against pertussis and promote immunopreventive strategies in pregnant women and household contacts of newborns.
The opinion of SAs should be taken into account in the decision-making process, and the MSSSI and the governments of the ACs should make a greater economic effort to fund a more comprehensive routine immunisation schedule for Spanish children, as opposed to a minimal one.
Another pressing issue is that the Spanish Agency of Medicines and Health Products (Agencia Española de Medicamentos y Productos Sanitarios [AEMPS]) is progressively blocking the use of some vaccines, such as the vaccine against rotavirus (Rotarix®), both vaccines against varicella (Varilrix® and Varivax®) and more recently the meningococcal group B vaccine (Bexsero®). All of them have been blocked without justification (the varicella and meningococcal group B vaccines have been restricted to “hospital-use only”), and Spain is the sole country in the world where these vaccines cannot be freely acquired in community pharmacies. This committee calls for the unrestricted availability of these vaccines, which are authorised by the European Medicines Agency (EMA), for their use by anyone who wishes to administer them after having received a prescription.
Since this document is restricted in length, we recommend consulting the online manual on vaccines of the AEP (“Manual de Vacunas en línea de la AEP”) which is updated on an ongoing basis and can be accessed in our website, www.vacunasaep.org, for extended information on every vaccine given to children and special circumstances pertaining to their administration, including any modifications to immunisation schedules, which have changed since those established in 2014 (especially in relation to the meningococcal group B and the human papillomavirus vaccines).
Vaccination against hepatitis B2015 Recommendation: We recommend vaccination in the first year of life with 3 or 4 doses of the monovalent and hexavalent preparations, or only the hexavalent preparation. Older children will receive 3 doses of the monovalent vaccine or of the combined hepatitis A and B vaccine.
In Spain, the annual incidence rate of hepatitis B has sustained the decline first seen in 2010, and was down to 1.49/100,000 inhabitants in 2013.1 Currently, all ACs vaccinate children in the first year of life, and over half of them administer the initial dose at birth.
If the child is vaccinated in the first year of life, any of the following schedules can be applied: at birth, 1, and 6 months; at birth, 2, and 6 months; at birth, 2, 4, and 6 months; and at 2, 4, and 6 months of age. All 4 schedules are appropriate for children of HBsAg-negative mothers. The first 3 are also appropriate for children of HBsAg-positive mothers, who should also receive 0.5mL of hepatitis B immune globulin, preferably within 12h of age.
If newborns are routinely vaccinated at birth, we recommend the administration of the hexavalent vaccine at 2 and 6 months, with a dose of the pentavalent preparation between them at 4 months of age.2
Vaccination against hepatitis B in previously unvaccinated older children and adolescents will consist of 3 doses at 0, 1, and 6 months.
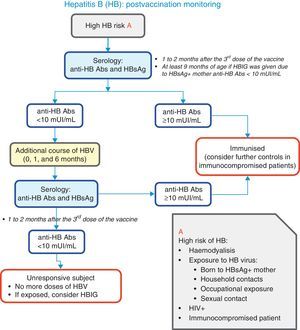
Fig. 2 presents a decision algorithm for the management of patients at risk for the disease.
Vaccination against diphtheria, tetanus, pertussis, poliomyelitis and Haemophilus influenzae type B2015 Recommendation: We recommend primary vaccination with the DTaP-IPV-Hib vaccine at 2, 4 and 6 months. The hexavalent vaccine (which includes the hepatitis B vaccine B [HBV]) or the pentavalent vaccine in combination with the HBV may be used. The first dose can be given earlier at 6 weeks of life. A booster dose of the DTaP-IPV-Hib should be administered at 15–18 months, one booster dose of DTaP or Tdap at 6 years, and one booster dose of Tdap at 11–12 years of age. Vaccination with a dose of Tdap is recommended in all pregnant women between weeks 27 and 36 of gestation, and in household members who will be in contact with the newborn (especially any postpartum mothers that were not vaccinated during pregnancy).
In Spain, the incidence of pertussis increased between 2010 and 2012 in all age groups, although the majority of complications occurred in infants (in 2012 there were 8 pertussis-related deaths in children younger than 3 months).3 Preventive strategies must prioritise the protection of this age group and a reduction in the incidence of pertussis in adolescents and adults.4 Since there is evidence of a quick decline in immunity, we recommend the administration of booster doses to adolescents and adults, especially if reduced-antigen preparations (Tdap) are used. Vaccination in the third trimester of gestation is safe and efficacious, and it is the most effective and efficient way to prevent pertussis in infants by means of the transplacental transfer of antibodies.5
Vaccination against meningococcal C2015 Recommendation: We recommend 3–4 doses of the monovalent conjugate vaccine (series of 1 [or 2]+1+1), with the following schedule: a first dose at 4 months (or two doses at 2 and 4 months, depending on the vaccine used), another at 12 months, and a last one at 12 years of age.
There is extensive evidence on the effectiveness of this vaccine.6
The rate of serogroup C invasive meningococcal disease (IMD) remains very low (0.15/100,000 inhabitants in 2011) in Spain. Since 2014 there is a new schedule recommended by the MSSSI and accepted by the ACs. The most salient change is the administration of 1 dose during adolescence, which the CAV-AEP has been advocating since 2013.
The prevalence of other serogroups (W135, Y and A) remains very low (3%) in Spain. The availability of quadrivalent conjugate vaccines (Menveo® and Nimenrix®) used only to vaccinate travellers to endemic regions offers an optimal alternative to the booster dose in adolescence, considering that travel to endemic countries becomes more frequent starting at this age.7
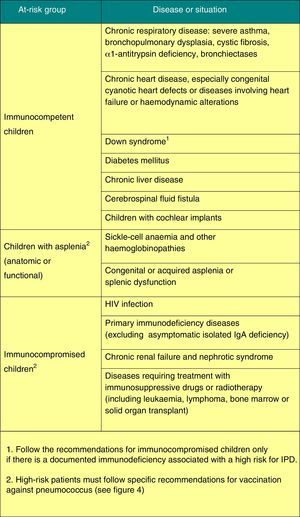
Vaccination against pneumococcal disease2015 Recommendation: Vaccination against pneumococcal disease is recommended for all children younger than 5 years and children that are immunocompromised or otherwise at risk at any age. In the absence of routine vaccination, the series should follow a 3+1 schedule. Based on epidemiological data for Spain, we recommend the 13-valent pneumococcal conjugate vaccine (PVC13).
There is growing evidence of the capacity of pneumococcal conjugate vaccines (decavalent [PVC10] and PVC13) to cause a marked reduction in the rate of invasive pneumococcal disease (IPD).8–10 Its impact on nasopharyngeal colonisation becomes significant when vaccination coverage reaches at least 65–75%, producing a marked herd immunity that results in a reduction of IPD in unvaccinated individuals too. So far there has been no evidence of serotype replacement with an increase of IPD caused by non-vaccine serotypes.
Both vaccines have a significant impact on noninvasive pneumococcal disease as well, having led to a reduction in the number of hospitalisations associated with pneumonia caused by pneumococcus or of any other aetiology, in vaccinated as well as unvaccinated individuals.9,11
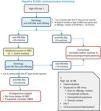
Figs. 3 and 4 show the groups at risk for pneumococcal disease and the recommendations for vaccination against pneumococcus.
2015 Recommendations: A first dose of MMR vaccine should be given at 12 months of age, with a second dose given between 2 and 3 years of age, preferably at 2, for the early correction of any potential primary vaccine failures.
Between July 2013 and July 2014, the number of reported cases of measles in the WHO European Region remained high, the number of rubella cases increased, and there continued to be outbreaks of mumps.12 Spain is among the countries with the lowest incidence rates for these diseases, but nevertheless shows the same trends.12 We recommend that an effort be made to maintain high coverage rates and pursue the eradication of these diseases.
The two available preparations of the MMR vaccine, Priorix® and MMRVaxpro®, are indicated for their use after 1 year of age, but they are authorised to start at 9 months if protection is required earlier due to specific epidemiological circumstances. Administration of a single dose at 12 months of age achieves seroconversion rates of 95% and greater for all three viruses.13 No monovalent or bivalent vaccines are available for these diseases, so the MMR has to be used for active immunisation against them.
Vaccination against human papillomavirus (HPV)2015 Recommendation: routine vaccination against HPV is recommended for all girls 11–12 years of age to prevent cervical cancer and premalignant lesions of the female genital tract.
The CAV-AEP estimates that the optimal age for vaccination is 11–12 years, as it improves vaccine acceptance, and predates the beginning of sexual activity.14 Unvaccinated women aged 13–26 years are a high-priority vaccination group and do not need to undergo a pap smear or a viral screening to be vaccinated. Fig. 1 shows the currently approved dosage for both vaccines.15
Data on the effectiveness of the vaccine in preventing persistent infection by HPV are already available, as well as data on the prevention of preneoplastic lesions caused by the most prevalent HPV types, including high-risk serotypes.16 It is likely that data on the prevention of cervical cancer and other types of cancer associated with HPV will become available in the years to come.
Both vaccines have an acceptable safety profile and a favourable benefit-cost ratio,17 yet the mean coverage in Spain does not exceed 75%,18 with higher coverage rates in ACs that have school-based vaccination programmes. Health professionals must make a greater effort to increase coverage.
The quadrivalent vaccine is approved for its use in boys and men15 and is included in the routine immunisation schedule of a few countries. The CAV-AEP is waiting for more data to be available before making a recommendation, but advises that male adolescents are made aware of this preventive measure.
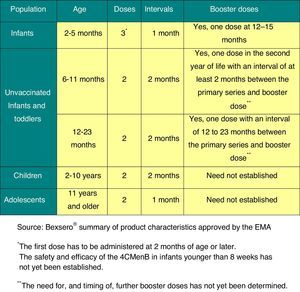
Vaccination against group B meningococcal disease2015 Recommendation: The profile of the serogroup B meningococcal vaccine makes it appropriate for inclusion in the schedules of all ACs, and it should be available for its unrestricted administration in all children at age 2 months.
Clinical trials of the only vaccine currently available for preventing IMD caused by serogroup B (Bexsero®)19 have demonstrated that it is immunogenic and safe in infants, children, adolescents, and adults, and that it induces immune memory.20
This vaccine has been approved by the EMA19 for its use starting at age 2 months, which authorises its unrestricted distribution in community and hospital pharmacies throughout the European Union. Some countries have already included this vaccine in their routine immunisation schedule. Particularly noteworthy is the decision of the United Kingdom to include it in their official schedule, starting with the vaccination of infants in a 2+1 schedule (at 2, 4, and 12 months).21
The MSSSI authorised this vaccine for immunisation against serogroup B meningococcus in August 2014, but categorised it as “hospital-use only,” restricting it to a very small group of patients, such as individuals with severe functional or anatomic asplenia, complement deficiency, or being treated with eculizumab; patients with a personal history of more than one episode of IMD, or laboratory personnel that manipulate meningococci in the course of their work. Its use has also been authorised to control outbreaks of IMD.22 These indications account for fewer than 5% of the overall IMD cases, so we consider that they are excessively limited. Over 95% of IMD cases occur in healthy individuals with no risk factors, especially in young children and adolescents.
The dosage for this vaccine is shown in Fig. 5.
Thus, the regulatory situation in Spain is quite peculiar as it does not allow professionals to freely prescribe a vaccine that has been approved by the EMA. Conversely, the vaccine will be freely distributed in the rest of Europe, and will even be part of the routine immunisation schedule of some countries and regions.
Vaccination against rotavirus2015 Recommendation: Vaccination against rotavirus (RV) is considered safe and recommended for all infants.
Since the introduction of the RV vaccines in 2006, the morbidity and mortality of gastroenteritis secondary to RV infection in infants and young children has decreased considerably,23 both in developing and developed countries.
In countries with routine vaccination there has also been a decline in morbidity and mortality in unvaccinated older children and adults.24 There has been strict post-marketing surveillance of the vaccine, with a special focus on intussusception, for which a low risk has been observed (approximately 1–5 cases per 100,000 vaccinated children).25 The benefits of this vaccine far outweigh the hypothetical risk of intussusception, as demonstrated by the data obtained in countries with routine vaccination, such as the United Kingdom.26 Thus, SCs and the WHO continue to recommend universal vaccination against RV.27
The pentavalent vaccine, RotaTeq®, continues to be the only one available in Spain. It is administered orally and can be given at the same time as other vaccines on the schedule. The dosage is shown in Fig. 1.
Vaccination against varicella2015 Recommendation: It is recommended that all children be vaccinated against varicella with 2 doses: one at 12 months and another at 2–3 years of age, preferably at 2. It is recommended that a two-dose catch up vaccination be done in children older than 2 that have not had the disease and are unvaccinated (or that the series is completed in children who have only received one dose previously).
In Spain, the two vaccines that are currently available (Varilrix® and Varivax®) have been classified as “hospital-use only” by the AEMPS.15 This decision, which has not been made in any other country in the world, is unjustified considering the high effectiveness of this vaccine in reducing the incidence of the disease and its complications, both in the vaccinated and unvaccinated populations,28,29 even when the coverage is less than optimal.30
These vaccines are generally well tolerated.31 The main concern in regards to widespread childhood vaccination is its potential epidemiological impact, shifting the disease toward older age groups or resulting in an increase in herpes zoster (HZ) cases in the general population. After nearly 20 years of routine vaccination in the United States, there has been no evidence of a shift in the burden of varicella to other age groups.29 There was an increasing trend in HZ even before childhood vaccination against varicella was implemented, and vaccination strategy does not seem to have any influence on the incidence of HZ.32,33 Both the WHO and the ECDC recommend the implementation of efficacious epidemiological surveillance measures so that studies on the cost efficiency of childhood vaccination against varicella can be performed.31,34
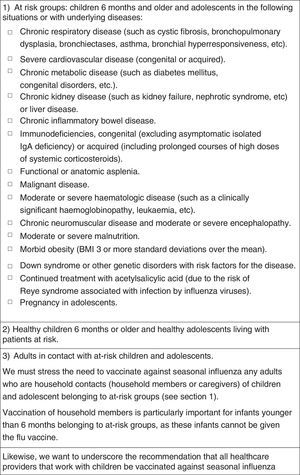
Vaccination against influenza2015 Recommendation: Vaccination against influenza is recommended for: (a) children 6 months and older and adolescents that are at risk due to specific circumstances or underlying disease, (b) children 6 months and older and adolescents that are healthy and living with at-risk individuals and (c) adults that are in contact with at-risk children and adolescents.
Fig. 6 summarises the recommendations of this Committee for vaccination against influenza, and Fig. 7 the dosage of the vaccine.35,36
At present, in Spain and with the available vaccines, universal childhood vaccination against seasonal influenza poses some concerns and drawbacks37: (1) the need to add a shot to the annual immunisation schedule, with the challenges for implementation and acceptability that this entails, (2) the low effectiveness of inactivated trivalent influenza vaccines in children younger than 2 years and (3) the cost would be high and there are not enough data on its efficiency in children in Spain. The introduction and increased availability of intranasal attenuated vaccines and quadrivalent preparations in Spain may change the current picture.
Additional information on this vaccine can be found in the document written yearly by this Committee before the start of each flu season.35
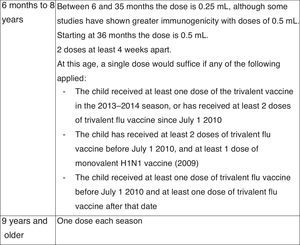
Vaccination against hepatitis A2015 Recommendation: Vaccination against hepatitis A with 2 doses separated by at least 6 months is recommended in specific risk situations. Administration of the vaccine should be considered for children older than 12 months attending child-care centres.
The different preparations, dosage, and indications of this vaccine for children and adolescents at risk are presented in Fig. 8.38
The vaccine has a 95% efficacy, and it is estimated that anti-HAV antibodies persist for at least 14–20 years in vaccinated children.39
Child-care centres that care for incontinent children are more likely to experience an outbreak of hepatitis A. Therefore, children older than 12 months attending child-care centres could benefit from vaccination.
Another special risk group are children born in Spain to immigrants from endemic regions, as they are at particular risk of contracting and then transmitting the disease when they visit their families in their country of origin.40
Conflicts of interest (last 5 years)DMP has collaborated in educational activities funded by GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD, as a researcher in clinical trials for Novartis vaccines, and as a consultant on Astra-Zeneca, Novartis and Pfizer advisory boards.
FJAG has collaborated in educational activities funded by GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD, and as a consultant on a Novartis advisory board.
JAF has collaborated in educational activities and as a researcher in clinical trials funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, and as a consultant on a Novartis advisory board.
MJCO has collaborated in educational activities funded by GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD, as a researcher in clinical trials for Pfizer, and as a consultant on a Novartis advisory board.
JMCR has collaborated in educational activities funded by GlaxoSmithKline, Novartis, and Sanofi Pasteur MSD.
NGS has collaborated in educational activities funded by Sanofi Pasteur MSD and has attended educational activities funded by Novartis and Pfizer.
AHM has received funding to attend domestic educational activities.
THSM has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, and as a researcher in clinical trials funded by GlaxoSmithKline and Pfizer.
MMM has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, as a researcher in clinical trials for GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, and as a consultant on a Novartis advisory board.
LOC has collaborated in educational activities funded by GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD, and as a researcher in clinical trials for GlaxoSmithKline.
JRC has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, and as a researcher in clinical trials for GlaxoSmithKline and Pfizer.
- -
David Moreno-Pérez (DMP). Infectología Pediátrica e Inmunodeficiencias, Unidad de Gestión Clínica de Pediatría, Hospital Materno-Infantil, Hospital Regional Universitario de Málaga. IBIMA Research Group. Departamento de Pediatría y Farmacología, Facultad de Medicina, Universidad de Málaga.
- -
Francisco José Álvarez García (FJAG). Paediatrician. Centro de Salud de Llanera, Asturias. Associate Professor of Health Sciences. Departamento de Medicina, Universidad de Oviedo.
- -
Javier Arístegui Fernández (JAF). Unidad de Infectología Pediátrica, Hospital Universitario de Basurto, Bilbao. Departamento de Pediatría, Facultad de Medicina, Universidad del País Vasco (UPV/EHU).
- -
María José Cilleruelo Ortega (MJCO). Servicio de Pediatría, Hospital Universitario Puerta de Hierro-Majadahonda, Madrid. Departamento de Pediatría, Facultad de Medicina, Universidad Autónoma de Madrid.
- -
José María Corretger Rauet (JMCR). Consell Assessor de Vacunacions, Departament de Salut, Generalitat de Catalunya (Barcelona).
- -
Nuria García Sánchez (NGS). Paediatrician. Centro de Salud Delicias Sur. Zaragoza. Associate Professor of Health Sciences. Departamento de Pediatría, Facultad de Medicina, Universidad de Zaragoza.
- -
Ángel Hernández Merino (AHM). Paediatrician. Centro de Salud La Rivota, Alcorcón, Madrid.
- -
Teresa Hernández-Sampelayo Matos (THSM). Servicio de Pediatría, Hospital General Universitario Gregorio Marañón. Departamento de Pediatría, Facultad de Medicina, Universidad Complutense de Madrid.
- -
Manuel Merino Moína (MMM). Paediatrician. Centro de Salud El Greco, Getafe, Madrid. Adjunct Professor. Facultad de Medicina, Universidad Europea, Madrid.
- -
Luis Ortigosa del Castillo (LOC). Servicio de Pediatría, Hospital Universitario Ntra. Sra. de Candelaria. Departamento de Pediatría, Facultad de Medicina, Universidad de La Laguna, Santa Cruz de Tenerife.
- -
Jesús Ruiz-Contreras (JRC). Servicio de Pediatría, Hospital Universitario 12 de Octubre, Madrid. Departamento de Pediatría, Facultad de Medicina, Universidad Complutense de Madrid.
Please cite this article as: Moreno-Pérez D, Álvarez García FJ, Arístegui Fernández J, Cilleruelo Ortega MJ, Corretger Rauet JM, García Sánchez N, et al. Calendario de vacunaciones de la Asociación Española de Pediatría: recomendaciones 2015. An Pediatr (Barc). 2015;82:44.e1–44.e12.
The members of Comité Asesor de Vacunas de la Asociación Española de Pediatría are presented in Appendix 1.