Edited by: Fernando Santos-Simarro. Molecular Diagnostic and Clinical Genetics Unit University Hospital Son Espases. Palma de Mallorca. Spain
Last update: January 2026
More infoGenomic medicine has made significant progress, driven by genomic technologies and their integration into clinical practice. However, its implementation presents challenges, particularly in genetic counseling (GC) and the interpretation of genomic data. Genetic counseling is a nondirective communication process aimed at helping patients and families understand and adapt to the implications of a genetic diagnosis. In pediatric care, GC takes on particular importance, adapting to the needs of each child development stage. Challenges arise with genetic newborn screening and rapid tests in intensive care units (ICUs) during the neonatal period. In children, GC focuses on the communication with the young patient and their family, addressing complex ethical issues such as consent, predictive testing and incidental findings. In adolescents, new challenges arise in relation to autonomy and decision-making. Multidisciplinary care is essential, including yet undiagnosed cases still on the journey commonly referred to as the “diagnostic odyssey”. This article reviews the role of GC across the stages of pediatric care in the framework of the current evidence on genomics.
La medicina genómica ha avanzado significativamente, impulsada por las tecnologías genómicas y su integración en la práctica clínica. Sin embargo, su implementación presenta desafíos, especialmente en el asesoramiento genético (AG) y en la interpretación de datos genómicos. El AG es un proceso de comunicación no directivo que busca ayudar a pacientes y familiares a entender y adaptarse a las implicaciones de un diagnóstico genético. En pediatría, el AG adquiere una relevancia particular, adaptándose a las necesidades de cada etapa del desarrollo infantil. Durante el periodo neonatal, se enfrentan retos en pruebas de cribado y diagnóstico rápido en unidades de cuidados intensivos neonatales (UCIN). En la infancia, el AG se centra en la comunicación con el paciente menor y su familia, abordando temas éticos complejos como el consentimiento, los estudios predictivos y los hallazgos incidentales. En la adolescencia se plantean retos en relación con su autonomía y la toma de decisiones. La atención multidisciplinar es clave, incluyendo casos sin diagnóstico a lo largo de la conocida “odisea diagnóstica”. Este artículo revisa el rol del AG en las diferentes etapas de la atención pediátrica en el contexto de los estudios genómicos actuales.
At present, what we know as genomic medicine is shaped by ongoing substantial technological advances, their integration in clinical practice and their central role in patient care. Its implementation poses certain challenges, particularly as regards genetic counseling (GC) and the interpretation of genomic data.
Genetic counseling is defined as a nondirective communication approach whose purpose is to help patients, partners and families with a genetic disorder to understand and adapt to its medical, psychological, familial and reproductive implications.1 While GC does not always entail genetic testing, GC is an essential component of any genetic test, such as exome or genome sequencing.
In the field of pediatrics, several aspects of GC are particularly important and give rise to unique dimensions that differentiate it from adult care. This article discusses GC in the context of genetic diagnosis from the neonatal period, through early and late childhood to adolescence, focusing on multidisciplinary care and the current status quo of genomic testing.
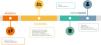
Genetic counseling in the different stages of child developmentThe genetic diagnosis process in pediatric patients has particular characteristics in each stage of child development, as the role of GC is adapted to the specific needs associated with it (Fig. 1). For instance, the neonatal period poses particular challenges in newborn screening, which is starting to include genetic techniques as first-tier tests, as well as GC in the context of rapid sequencing in neonatal intensive care units (NICU).2,3 During childhood, the GC process chiefly focuses on communicating with and supporting children and their parents or legal guardians in a family-centered approach.4 The needs or interests of the parents can add to or interfere with those of the minor so that, in some instances, issues such as consent/assent, findings unrelated to the reason for testing (incidental or secondary findings) and genetic testing in unaffected individuals (predictive testing) become ethically complex.5,6 The need to communicate with pediatric or adolescent patients and the differential approach to GC depending on the stage of development add a distinctive dimension to the genetic counselor’s role, which requires specific knowledge and skills.
During these stages, a multidisciplinary care approach and integrated care teams including clinical geneticists, genetic counselors, case managers and other specialists are crucial for diagnosis and in supporting families.
Neonatal periodNewborn screeningNewborn screening allows the early detection and identification of treatable diseases in newborn infants based on biochemical markers, frequently before the onset of symptoms. It is a secondary prevention public health measure that allows prompt intervention with the aim of reducing morbidity, mortality and potentially severe complications.7 However, there are many diseases in which early detection could change patient management and outcomes for which there is no known specific biomarker but that could be diagnosed through a genetic study.
In recent years, different initiatives have been launched to include genome sequencing (GS) in newborn screening programs, the implementation of which poses certain challenges at the medical/patient care, bioethical, social and familial levels that need to be evaluated before its potential introduction.3,6,8,9 Studies conducted at the international level have explored aspects related to the potential impact on parents and parental attitudes in relation to the use of GS in newborn screening, revealing that most parents find it acceptable. However, preferences vary between families as to the types of results they wish to know about, ranging from treatable or preventable diseases with onset in childhood to diseases with onset in adulthood that cannot be prevented or treated. Many of these studies reflect the concerns of parents, evincing the need for informed consent prior to testing as well as delivery of adequate GC.10–12 Thus, at this time, additional evidence is still required to define the role of GC in this setting, the needs of families and the ethical and health care implications of introducing GS in public health programs.
Rapid diagnosis at the NICUIf we consider the earliest key time points at which a genetic diagnosis may be made in a neonate, one would be in the context of the evaluation performed at admission to a NICU. This particular scenario, characterized by a high acuity and urgency in clinical decision-making, poses various challenges in undertaking GC with the family.2,13
In GC, one of the key elements for building trust and rapport with the patient and family is striving to set up an appropriate physical environment and pace for the sessions. The NICU setting can be highly medicalized and is associated with frequent interruptions by other members of the care team. Frequently, due to the spaces where GC is delivered, conversations with the family are held standing or at the bedside. This stands in sharp contrast to customary practice in GC clinics, where more time is devoted to gathering information through the review of health records and the medical literature in addition to communicating with the medical providers in charge of the patient.13,14
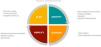
Even considering these factors, it is possible to deliver effective GC in the NICU with approaches based on rapid or crisis counseling models (Fig. 2). Since it may not be possible to address or explore some important psychosocial and emotional aspects in a single encounter, it is important to schedule additional follow-up sessions.2,13
Neonatal intensive care unit rapid genetic counseling and psychological assessment model. Adapted from Ayres et al (2019).13
In this context, the diagnostic process tends to include trio testing (of samples from the patient and both parents), which requires anticipating any potential findings and direct repercussions for parents or families. Although, in the face of a high-acuity situation, the priorities of families tend to focus on testing that can guide the management and help address the needs of the newborn, it is important to inform them of all the characteristics and limitations of the test as well as the potential for incidental findings.15,16
ChildhoodGenetic counseling in the context of diagnosisOne of the key aspects in GC sessions is the communication with the patient, especially when the patient is a child or adolescent. Certain cross-cutting skills and considerations that apply to different areas of pediatric care can be used to facilitate GC. The counselor should consider the different stages of social, cognitive and emotional development (Appendix A supplemental table) and adapt the specific approach and activities used during sessions to facilitate communication (Fig. 3). Some of these skills entail adapting language to the age and maturity of the minor (“speaking the patient’s language”). To do so, it is helpful to listen carefully to the words the patient uses to define a given concept and use similar vocabulary. On the other hand, the use of specific activities in the session can help the child share information through play or to experience or express specific feelings through drawing or construction.17 Counseling children with neurodevelopmental disorders requires particular consideration and sensitivity. Creating a safe space where the young patient feels comfortable in exploring feelings and being him or herself facilitates the GC process.8
Specific skills and activities useful in genetic counseling sessions.17
During the GC process, the counselor assesses the medical, psychosocial and emotional needs of the patient and family. However, in the context of pediatric care, the boundaries between the family’s and the patient’s needs can be difficult to determine. The role of the counselor is complex and key in recognizing, validating and responding to the needs of the parents which, at times, come into conflict with the duty to safeguard the autonomy of the child. Part of the counseling process is finding a balance between the aspects that affect the rights of the minor and supporting the needs of the family. The development of rapport early in the process is key for the counselor and the rest of the care team to have a good relationship with the patient and family. This rapport and trust will allow delivery of individualized family-centered care throughout the care episode.
In any encounter with children and adolescents, the counselor needs to keep in mind that they neither grow nor make decisions in isolation from the family environment, as they are part of a system of relationships that will be affected by the decisions and results of a diagnostic process. It may be difficult to understand the situation of the minor without considering the context of the minor’s life and the dynamics of the family as a unit that should be the focus of professional intervention.4
In some instances, in the process of deciding which tests to perform in the child, parental wishes may come into conflict with good clinical practice in GC.19–22 Parents often wish to know the carrier status of their healthy children, especially if a diagnosis has already been made in a close relative. However, genetic testing is generally discouraged in unaffected minors, and it is only actually indicated for diseases with onset in childhood in which it would be medically beneficial to make an early diagnosis and for which therapeutic intervention is currently available. Exploring the motivation of parents in pursuing testing, explaining the rationale of clinical guidelines and establishing a plan to offer carrier screening to siblings once they can make an informed decision themselves can help reassure parents. The maturity and preferences of the minor, the concerns of the parents and the reproductive implications for the child are among the factors that genetic health professionals must take into account in reaching an agreement with the family.23
It is also important to remember the role of siblings and the impact the diagnosis may have on them. Some authors have documented the experiences of adolescent siblings of patients with rare diseases, highlighting the impact on the development of their own identities and the uncertainty they experience, among other feelings, during the diagnosis and in relation to their future health.24 Siblings are usually present and play an important role in GC visits and family discussions; failing to specifically address their concerns or needs may lead to misinterpretation and exacerbate their feelings of uncertainty. In addition, during adolescence and, especially, in adulthood, siblings tend to take on some of the responsibility in supporting and caring for the patient. The scope of practice of genetic counselors includes educating parents and underscoring the importance of including siblings and informing them of the implications of a genetic diagnosis, providing the necessary support to address their concerns, feelings and doubts.25,26
AdolescenceAdolescence is a transitional stage between childhood and adulthood.27 During this stage, challenges may emerge in relation to the development of autonomy, peer relationships, behavior and decision-making.28,29
One of the key challenges in GC with adolescents is developing rapport, in addition to adequately assessing their level of maturity, finding the way to engage them in the process and determining when and how to include the parents.18,30
Although few studies have focused on the perspective of adolescents in this context, some authors have documented their experience in GC, assessing stage-specific factors that emerge around the diagnosis of a genetic disorder. Having a period of “warming up” prior to GC, offering the option of holding individual sessions without the parents, respecting their maturity, speaking their language, avoiding a paternalistic attitude and normalizing the diagnosis are among the aspects to consider in the practice of GC.31
Making adolescents to feel heard and in control in the session, allowing them to decide which information to receive and who should be present, helps create a safe space for them to express feelings, doubts and concerns.4 During this stage, it is important for providers to be mindful of the adolescent’s role as the primary target of intervention in ensuring comprehension of the information and to engage in decision-making. In cases in which genetic diagnosis takes place during childhood, when GC most likely focused on the parents, it is important to ensure that the counseling process can resume once the patient reaches adolescence, before adulthood.
Informed consentIn the context of genetic testing, the informed consent (IC) process has different characteristics and involves different parties depending on the stage of development.
Informed consent is not only an ethical and moral concept, but also a legal one, and true informed consent entails that the patient has the capacity to make decisions, is free to make those decisions, and understands the provided information. During childhood, consent is not provided by the patient or minor, but by the parents or legal guardians.32
Notwithstanding, providers need to consider the opinions of the patient, engaging the minor throughout the decision-making process (assent), taking into account the level of maturity and the stage of development at which it is taking place. In Spain, based on the mature minor doctrine (ages 12–15 years), the IC form must be signed by the parents or legal guardians with the assent of the minor. When it comes to health care, the age of majority is 16 years, which is when patients can start to exercise their autonomy by giving IC.33
The increasing use of genetic tests, especially GS, poses new challenges for health care professionals in relation to IC. It increases the complexity of IC due to the possibility of incidental findings and greater uncertainty due to the identification of variants of uncertain significance.16
Inevitably, the amount and complexity of genomic results has been increasing, and it has become difficult to address them in detail in a single pre-test GC session. One of the main challenges involves the appropriate amount of information to be delivered, and how to ensure comprehension. Aspects like the reclassification of variants, the possibility of incidental or secondary findings and the ES reanalysis pathway in cases in which a diagnosis was not reached are among the most important to be covered in the discussion held prior to testing and for the purposes of informed consent.
Genetic counselors play a key role in helping patients understand results and potential implications, risks and benefits, in addition to providing support in navigating the uncertainty and complexity associated with genetic testing. The integrator of these professionals in multidisciplinary care teams is essential, especially in light of the emerging application of genomic sequencing to clinical practice.34
Implications of genetic testing: secondary and incidental findings and carrier statusThe implementation of genomic techniques has increased the frequency of results of uncertain clinical significance, detection of carrier status for recessive diseases, secondary findings (opportunistic screening for a preset list of genes for preventive purposes unrelated to the presenting complaint) or incidental findings (unexpected pathogenic variants in genes that were not actively targeted).
In pediatric care, the age of onset of the diseases associated to the detected variants, and whether or not the findings are actionable are key aspects in establishing criteria regarding which findings to disclose and which to withhold. However, there is variation among scientific societies in the field of genetics across the world regarding the indication of active search for secondary variants and the disclosure of incidental findings or carrier status for recessive diseases in underage patients (Table 1).20,35–40 At present, the concept of “actionable” findings and the difference between secondary and incidental findings are not precisely defined. At present, there is no global consensus and the subjects continues to generate considerable interest and debate.34
Positions of different scientific societies regarding incidental and secondary findings and detection of carrier status in genetic testing of minors.
| Secondary findings | Incidental findings | Carrier status | |
|---|---|---|---|
| AEGH32 (Asociación Española de Genética Humana) |
|
|
|
| ESHG36 (European Society of Human Genetics) |
|
|
|
| ACMG37 (American College of Medical Genetics) |
|
|
|
| AGNC38 and BSGM20 (Association of Genetic Nurses and Counsellors- UK) (British Society of Genetic Medicine) |
|
|
|
| SFMPP39 (Société Française Médecine Prédictive Personnalisée) |
|
|
|
| CCMG40 (Canadian College of Medical Geneticists) |
|
|
|
Abbreviations: GC: genetic counseling; IC: informed consent; N/A: not available.
In its current recommendations document, the European Society of Human Genetics (ESHG) proposes the development of projects and initiatives to better understand the impact and perceptions of the potential implementation of opportunistic genomic screening.36
Several studies on the subject have assessed the impact and usefulness of various opportunistic screening approaches in adults and children. Different informed consent models and approaches to the search for secondary findings in two or more steps or stages (that is, after disclosing the primary findings) have been proposed and evaluated in some of these studies, contributing experiences and information worth considering by scientific societies in their review of recommendations and future protocols.41,42
In this context, pretest and post-test counseling by genetic health specialists is essential and is particularly important for any patient undergoing genetic testing.
ConclusionThe integration of genomic medicine in clinical practice poses novel challenges on account of the greater complexity of the interpretation and repercussions of genetic tests results. This calls for multidisciplinary care teams, which should include genetic counselors specialized in pediatric care to support decision-making and communication with the patient and family.
FundingThis research did not receive any external funding.
The authors have no conflicts of interest to declare.