To evaluate bone mineral density (BMD) in children with sickle cell disease (SCD) in the Community of Madrid.
Materials and methodsThe BMD was estimated in 40 children with SCD, and with an age range between 3 and 16 years, using densitometry (DXA), as recommended by the International Society for Clinical Densitometry (ISCD).
ResultsThe mean age at the time of the study was 7.97±3.95 years, the mean value of the DXA expressed in Z-score was −0.91±1.46 with a range of minimum values −5.30 and 2.30 maximum. More than half (57.5%) of all the children had normal BMD (Z>−1), 25% had low BMD (Z between −1 and −2), and 17.5% showed abnormal Z-score values of osteoporosis (Z-score<−2). The Pearson linear correlation was statistically significant between Z-score value and the haemoglobin level (r=0.368, p=.019), finding no correlation with the levels of 25 (OH) vitamin D.
ConclusionProspective studies are needed with a larger number of patients to understand the future implications of bone densitometry changes and associated risk factors.
Evaluar la densidad mineral ósea (DMO) en niños con enfermedad de células falciformes (ECF) de la Comunidad de Madrid.
Material y métodosSe valora la DMO en un total de 40 niños con ECF y rango de edad entre 3-16 años, mediante densitometría (DEXA) siguiendo las recomendaciones de la Sociedad Internacional de Densitometría Clínica (ISCD).
ResultadosLa edad media en el momento del estudio fue de 7,97±3,95 años; el valor medio de la DEXA expresado en Z-score es de -0,91±1,46 con un rango de valores mínimo de -5,30 y máximo de 2,30. Un 57,5% de los niños tiene DMO normal (Z>-1), un 25% tienen DMO baja (Z entre -1 y -2) y un 17,5% presentan Z-score patológico con valores de osteoporosis (Z-score<-2). Los estudios de correlación solo encuentran una correlación lineal de Pearson significativa estadísticamente entre valor de Z-score y valor de Hb (r=0,368, p=0,019), no encontrando correlación con los niveles de 25 (OH) D.
ConclusiónSe necesitan estudios prospectivos, con mayor número de enfermos para conocer las implicaciones futuras de la densitometría alterada y los factores de riesgo asociados.
In recent years, bone mass alterations or low bone mineral density has been the subject of ongoing attention. The peak bone mass acquired by the end of maturation is a good predictor for the risk of future fractures. Clinicians are generally familiar with the terms “osteopaenia” (decrease in bone mass) and “osteoporosis” (a more severe loss of bone mass associated with a greater risk of fracture).1–3 The diagnosis of osteoporosis in children is defined as low bone mineral density (BMD) and a fracture history with long bone fracture of the lower extremities, vertebral compression fracture, or two or more long bone fractures of the upper extremities.1
The earliest studies that analysed the role of bone health in fractures in the paediatric age group were conducted in New Zealand.4,5
A prospective study in healthy African children 5–9 years of age that analysed the relationship between vitamin D and fractures concluded that a significant number of children with fractures also have low vitamin D levels (59%).6 Vitamin D deficiency seems to play a previously unknown role in fractures in the paediatric age group.
Furthermore, there is evidence that vitamin D supplementation in healthy children and adults with deficiency leads to a significant increase in BMD, decreasing the risk of fractures.7,8
Children with sickle cell disease (SCD) often develop bone complications manifested as vaso-occlusive bone pain crises, dactylitis, osteomyelitis, avascular necrosis or vertebral deformity. In this population, the literature describes a decrease in BMD secondary to chronic anaemia and bone marrow hyperplasia and associated with a higher risk of osteopaenia and osteoporosis.9,10
Recent studies show that children with SCD frequently have severe vitamin D deficiency. For instance, a cross-sectional study of 78 children with SCD performed in our hospital found that only 20.5% had 25-hydroxyvitamin D (25(OH)D) levels above 30ng/mL, the threshold considered optimal for bone health.11
Despite the higher risk of bone disease in this population, there is little information on BMD in children with SCD and its association with 25(OH)D levels and the risk of fractures. Our study assessed the prevalence of BMD abnormalities in children with SCD from the autonomous community of Madrid using densitometry (dual-energy X-ray absorptiometry [DXA])12,13 and analysed potential risk factors associated with low BMD.
Materials and methodsWe conducted a cross-sectional study between October 2009 and February 2011 in a cohort of 120 children with SCD residing in the autonomous community of Madrid and who were being followed up at the paediatric haematology department of the Hospital Gregorio Marañón (HGUGM) to evaluate BMD in this population by means of DXA.
We included a total of 40 patients, starting at age 3–4 years to avoid the need for sedation during DXA. We included all possible patients who made at least one visit to the hospital during the study period. We excluded children known to have conditions affecting growth or nutritional status, with chronic liver or kidney function abnormalities, undergoing treatment with drugs that affect the skeleton, or who had had SCD complications the previous month (vaso-occlusive crisis, fever, acute chest syndrome).
The study was approved by the board of ethics of our hospital, and we obtained the signed informed consent from all participants.
We collected the demographic and anthropometric data in the course of routine follow-up visits to a physician in the team.
Bone mineral density was assessed by means of DXA. We measured BMD at the posterior–anterior spine, and not at the hip and proximal femur due to the great variability of the latter areas during growth, adhering to the International Society for Clinical Densitometry (ISCD) recommendations for children. The instrument used for this study was a LUNAR DPX-IQ 5539 machine that met the requirements for research in paediatrics. The results were interpreted by an experienced DXA radiologist and expressed as Z-scores, that is, the number of standard deviations (SDs) from the mean BMD of a child compared to other children of the same age, sex and ethnicity. Z-scores below −2 SD were considered abnormal. Low BMD or osteopaenia was defined as a Z-score between −1 and −2 SDs, and a normal BMD as a Z-score above −1 SD. Osteoporosis was not defined on the sole basis of an abnormal Z-score in any instances. Children were only diagnosed with osteoporosis if the following two conditions were met: low BMC and a fracture history with long bone fracture of the lower extremities, vertebral compression fracture, or two or more long bone fractures of the upper extremities.1
Other laboratory tests were performed on the same day as the DXA scan. Haemoglobin, reticulocytes and calcium and phosphorus levels were measured with standard laboratory techniques. Vitamin D levels were assessed by quantitative determination of 25(OH)D using the LIASON 25 OH Vitamin D TOTAL Assay (310600). Vitamin D deficiency was defined as a 25(OH)D level below 20ng/mL (50nmol/L). Vitamin D insufficiency was defined as a 25(OH)D level between 21 and 29ng/mL (52.5–72.5nmol/L) and a normal vitamin D level as a 25(OH)D level equal or above 30ng/mL (75nmol/L). We also defined hyperparathyroidism as a serum parathyroid hormone (PTH) concentration above 50ng/L. We measured osteocalcin by immunoradiometric assay (IRMA) and bone alkaline phosphatase (BAP) by the Access Ostase immunoenzymatic assay.
Statistical analysisAll the data were entered in an SPSS statistics 18 database and analysed using the same application. Descriptive statistics were used to summarise population data, and measures of central tendency to describe quantitative variables. We used Student's t-test and ANOVA to compare parametric distributions and the Mann–Whitney U-test and the Kruskall–Wallis test for nonparametric distributions. We expressed qualitative variables as frequencies and percentages, and compared them by means of the chi square test, Fisher's exact test or McNemar's test depending on the samples. We analysed correlation by means of Pearson's and Spearman's correlation coefficients. We also performed simple and multiple regression analysis.
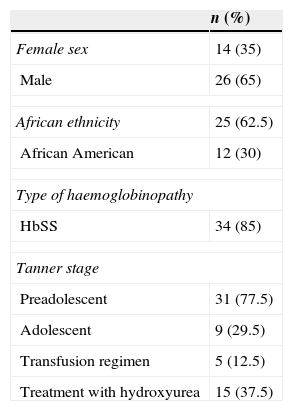
ResultsTable 1 summarises the descriptive statistics results for patients who underwent DXA for bone mass assessment.
Summary of epidemiological and laboratory data and Z-scores for the group of children with sickle cell disease (n=40) whose bone mineral density was assessed by densitometry (DXA).
| n (%) | |
|---|---|
| Female sex | 14 (35) |
| Male | 26 (65) |
| African ethnicity | 25 (62.5) |
| African American | 12 (30) |
| Type of haemoglobinopathy | |
| HbSS | 34 (85) |
| Tanner stage | |
| Preadolescent | 31 (77.5) |
| Adolescent | 9 (29.5) |
| Transfusion regimen | 5 (12.5) |
| Treatment with hydroxyurea | 15 (37.5) |
| N | Range | Mean±DS | Variance | |
|---|---|---|---|---|
| Breastfeeding (months) | 40 | 0–9 | 4.50±3.00 | 9.02 |
| Vitamin D prophylaxis (months) | 13 | 2–24 | 8.54±6.24 | 38.93 |
| Hospitalisation (days/year) | 37 | 0–2 | 0.24±0.64 | 0.41 |
| Age (years) | 40 | 4–16 | 7.97±3.95 | 15.66 |
| DXA results | ||||
| Z-score | 40 | −5.30–2.30 | −0.91±1.46 | 2.15 |
| Laboratory tests | ||||
| Hb g/dL | 40 | 5–11.30 | 8.74±1.36 | 1.87 |
| Reticulocytes | 37 | 75–355.600 | 119,470±101,919.34 | 10,387,553,157.52 |
| Total calcium mg/dL | 38 | 7.40–10.30 | 9.39±0.52 | 0.27 |
| Ionised calcium mmol/L | 36 | 0.64–1.30 | 1.07±0.12 | 0.016 |
| Mg mg/dL | 40 | 1.70–3.00 | 2.18±0.26 | 0.068 |
| P mg/dl | 39 | 4–6.50 | 5.36±0.57 | 0.326 |
| PTH ng/L | 39 | 6–185 | 65.33±47.61 | 2267.22 |
| Total AP U/L | 36 | 64–461 | 199.75±79.40 | 6305.45 |
| BAP μg/L | 30 | 19–115 | 57.93±26.63 | 712.06 |
| Osteocalcin μg/L | 27 | 1–10 | 3.74±2.76 | 7.64 |
| 25(OH)D ng/ml | 40 | 4–44.50 | 16.89±8.28 | 68.58 |
| CRP mg/L | 30 | 0.10–2.60 | 0.44±16.89 | 0.29 |
AP: alkaline phosphatase; BAP: bone alkaline phosphatase; CRR: C reactive protein; Hb: haemoglobin; Mg: magnesium; P: phosphorus; PTH: parathyroid hormone; SD: standard deviation.
We analysed data for a sample of 40 children that underwent DXA between October 2009 and September 2011.
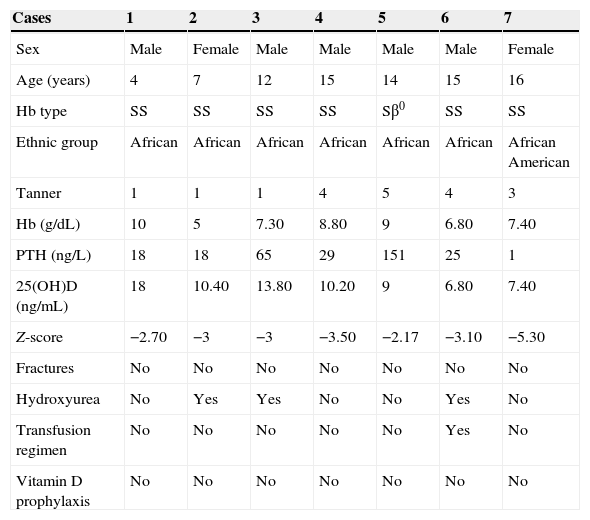
Table 2 presents the main data pertaining to the children with abnormal DXA results.
Data of the children with a pathological densitometry result.
| Cases | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Male | Male | Male | Female |
| Age (years) | 4 | 7 | 12 | 15 | 14 | 15 | 16 |
| Hb type | SS | SS | SS | SS | Sβ0 | SS | SS |
| Ethnic group | African | African | African | African | African | African | African American |
| Tanner | 1 | 1 | 1 | 4 | 5 | 4 | 3 |
| Hb (g/dL) | 10 | 5 | 7.30 | 8.80 | 9 | 6.80 | 7.40 |
| PTH (ng/L) | 18 | 18 | 65 | 29 | 151 | 25 | 1 |
| 25(OH)D (ng/mL) | 18 | 10.40 | 13.80 | 10.20 | 9 | 6.80 | 7.40 |
| Z-score | −2.70 | −3 | −3 | −3.50 | −2.17 | −3.10 | −5.30 |
| Fractures | No | No | No | No | No | No | No |
| Hydroxyurea | No | Yes | Yes | No | No | Yes | No |
| Transfusion regimen | No | No | No | No | No | Yes | No |
| Vitamin D prophylaxis | No | No | No | No | No | No | No |
Hb: haemoglobin; PTH: parathyroid hormone.
The analysis of the association between age at the time of the DXA scan and Z-score values showed that the median age was below 7 and a half years in children with a normal or low BMD (n=33), while the median age of children with an abnormal BMD was higher, 12 and a half years (n=7).
The correlation analyses for the association between the Z-score and other quantitative variables only found a statistically significant linear Pearson correlation between Z-score values and Hb levels (r=0.368; P=.019), that is, children with higher Hb levels had higher Z-scores.
We found no correlation with the remaining quantitative variables we analysed: total calcium, ionised calcium, magnesium, phosphorus, alkaline phosphatase, BAP, PTH, osteocalcin, 25(OH)D, iron and ferritin levels, age at the time of the DXA scan, duration of breastfeeding in months, duration of vitamin D prophylaxis in months, and number of hospitalisation days per year.
The simple and multiple regression analyses that we performed did not find a linear association or an explanatory model (cause-effect) between the various quantitative variables and the Z-score values.
The Mann–Whitney's U nonparametric test did not find significant differences in the comparison of the DXA results expressed as Z-scores between different groups of children classified by sex (P=.21). The same test also found no differences in the Z-scores of the group that received prophylaxis in the first year of life compared to the group that did not (P=.65), nor in the group that received hydroxyurea versus the group that did not (P=.467). The nonparametric Kruskal–Wallis test also showed no significant differences (P=.45) between different ethnic groups or between patients with different types of SCD (P=.28).
The analysis of the categorical variables by means of the chi square test or Fisher's exact test did not find significant results in the comparison of abnormal Z-scores with 25(OH)D levels below 20 and 30ng/L, respectively.
The chi square test for the comparison of the Z-scores of patients who were treated with hydroxyurea and of patients who were not showed that 36% of the latter had abnormal Z-scores. This percentage reached 53.3% in the group treated with hydroxyurea, but the difference was not statistically significant (P=.336). There were also no statistically significant differences (P=.531) between children with and without hyperparathyroidism (39.1% vs 50%), or between sexes (28.6% in girls vs 50% in boys; P=.191). Eighty percent of the children treated with a transfusion regimen had normal DXA results, and 20% had abnormal results (P=.373).
DiscussionWe conducted a cross-sectional observational study to assess bone health by means of DXA in children 3–16 years of age (n=40). Z-scores were abnormal in some patients younger than 5 years, so we could not clearly define the cut-off age at which DXA results start to be significantly abnormal, although most patients in the abnormal Z-score group (Z<−2), which amounted to 17.5% of the sample, were older than 10 years. This finding is consistent with the ISDC recommendation of starting to take DXA measurements at 10 years of age, as proposed for other haemoglobinopathies like thalassaemia major.14
The studies conducted in children with SCD to assess bone mass by means of DXA have had a cross-sectional design, small samples, and insufficient data to evaluate the technique in this population and in individuals 9 years of age and older in general.15,16 They concluded that poor bone mineralisation probably starts at earlier ages, and that further research should be devoted to the impact of correcting insufficient or deficient levels of calcium and vitamin D.
The most interesting study is the one conducted by Buison et al.17 It was a case–control study of 90 children with SCD (HbSS phenotype) 4–19 years of age that were compared to 198 healthy children of similar characteristics. They found a significantly lower BMD, assessed by DXA, in children with SCD (P<.0001).
Among all the analysed variables, our study only found a linear correlation between the Z-scores and haemoglobin levels. This may be explained by a decrease in erythropoiesis in children that have higher haemoglobin levels and lesser degrees of haemolysis, which prevents bone marrow hyperplasia and the subsequent cortical thinning. This finding is consistent with the work of Buison et al.17 and has also been described in Brazilian adults with SCD.18
Similarly, a retrospective study of 103 adults with SCD by Sarrai et al.19 found a high prevalence of altered bone density (79.6%) assessed by means of DXA. The study did not find an association with age, but found an association with haemoglobin levels, as low Hb was a predictor for abnormal bone density.
Some authors have also proposed that the level of 25(OH)D is a factor that influences BMD in patients with SCD. In this regard, a study conducted in adults in Saudi Arabia that compared SCD patients with a healthy population of similar characteristics described a significantly lower BMD in the group of affected patients compared to the control group (P<.001 for measurements at both the spine and the femur). The prevalence of osteoporosis in patients with SCD and vitamin D deficiency is 73.8% (45/61) in male patients and 92.4% (85/92) in female patients, while in the control group the prevalence of osteoporosis was 30% in men and 63.6% in women. This study did not analyse other causes of low BMD, such as hypogonadism, altered sex hormones or iron overload.20
Our study did not find a correlation between 25(OH)D levels and BMC measured as a Z-score, a finding that is consistent with studies conducted in children with SCD.21 Understanding this fact would require prospective studies with repeated measurement of 25(OH)D levels.
The risk of osteoporosis and osteopaenia in paediatric patients with SCD has not been properly established yet either, and at present we do not know the threshold for abnormal DXA values. There are studies in adults that demonstrate that adults with SCD are at increased risk of osteopaenia and osteoporosis. A study of 36 adults conducted in Saudi Arabia found that 82% of the men and 76% of the women had osteoporosis or osteopaenia.22 This study was later expanded to 87 patients, showing a prevalence of osteopaenia and/or osteoporosis in 65% of male patients and 65.2% of female patients.23
A few studies of healthy adults have analysed the association between 25(OH)D levels and bone health and obtained disparate results.24,25 Many observational studies in adults have sought to determine the threshold level of 25(OH)D beyond which there is increased risk of fracture. Some studies proposed levels below 16ng/mL, while others placed the threshold at 20ng/mL.26–30
There are very few published studies on the subject of fractures in SCD patients. Most of them are isolated case reports, and one is a study of a cohort of children with osteomyelitis and SCD.31–33
Paradoxically, individuals of African descent without SCD, despite having a higher 25(OH)D deficit than Caucasian individuals of similar characteristics, have a lower risk of bone fracture due to a greater bone mass (10–15% greater than other ethnic groups).34 We do not know whether this advantage is maintained in the SCD population.
Although many questions have yet to be resolved and the current literature has not established a threshold for abnormal DXA results in children, studies in adults and children published in 2011 and 2012 have described an association between vitamin D deficiency and a greater risk of fractures, low BMD and chronic musculoskeletal pain.35–40
The results of our study are limited by the sample size, it being a single-centre study, and the lack of a control group.
ConclusionsWhile our study did not determine the age from which alterations in BMC develop in children with SCD, it suggested the presence of a low BMD from 10 years of age, with abnormal DXA results starting at age 12 years. At present, there are no evidence-based recommendations on when to assess BMD and 25(OH)D levels in this population. Still, the development of a strategy to improve peak bone mass and prevent fractures and osteoporosis would probably be beneficial. It is essential that we intervene during childhood, and in this sense it would be worth considering the serial measurement of vitamin D levels and their subsequent correction.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Garrido Colino C, Beléndez Bieler C, Pérez Díaz M, Cela de Julián E. Evaluación de la densidad mineral ósea en pacientes con enfermedad de células falciformes. An Pediatr (Barc). 2015;82:216–221.