Mycoplasma pneumoniae (MP) is one of the most common etiological agents of community-acquired pneumonia (CAP) in children. We aimed to describe the clinical and epidemiological characteristics, treatment and outcome of children diagnosed with community-acquired MP pneumonia (CAMP) in a tertiary hospital in Valencia, Spain.
Material and methodsMedical records of children <14 years with CAMP were retrospectively reviewed from January 2010 to December 2015. Patients with radiological evidence of pneumonia and microbiological confirmation of MP (PCR from nasopharyngeal swab and/or serum specific IgM) were considered CAMP.
ResultsOne hundred and sixty two children were diagnosed with CAMP; median age 6 years (IQR: 4–9). The positive MP test rate among children with CAP progressively increased with age as did the empirical use of macrolides. There were two peaks of cases in 2011 and in 2015, being July, August, November and December the seasons with the higher number of cases. The most frequent radiological pattern was segmental infiltrate (62.3%) and 22 (13.6%) children had pleural effusion. It was noteworthy the mild symptomatology and low levels of inflammatory parameters that children with CAMP had. A macrolide was empirically initiated in 68.5% of cases. Hospital admission rate was inversely proportional to patient's age.
ConclusionsAccording to this study, older, less symptomatic patients and with lower inflammatory parameters had the greatest rate of MP infection among children with CAP and thus they could benefit of empiric macrolide therapy. Therefore, knowing the epidemiology of a geographical area may be important for the management of CAP in children.
Mycoplasma pneumoniae (MP) es uno de los agentes etiológicos más comunes de las neumonías adquiridas en la comunidad (NAC) en niños. Objetivo: describir las características clínicas y epidemiológicas, tratamiento y evolución de los pacientes con NAC por MP (NACM) en un hospital terciario de Valencia, España.
Material y métodosSe revisaron retrospectivamente las historias clínicas de los niños<14 años con NACM entre enero de 2010 y diciembre de 2015. Los pacientes con evidencia radiológica de neumonía y confirmación microbiológica de MP (PCR de exudado nasofaríngeo y/o anticuerpos IgM específicos frente a MP) se consideraron NACM.
ResultadosUn total de 162 pacientes se diagnosticaron de NACM; mediana de edad de 6 años (rango intercuartílico: 4-9 años). La proporción de pruebas positivas para MP en pacientes con NAC, así como el uso empírico de macrólidos, aumentó progresivamente con la edad. Hubo un pico de casos en 2011 y en 2015, con un máximo de casos en julio, agosto, noviembre y diciembre. El patrón radiológico más frecuente fue el infiltrado segmentario (62,3%), mientras que 22 (13,6%) presentaron derrame pleural. Los niños con NACM desarrollaron una clínica leve, con poca elevación de parámetros inflamatorios. Se inició tratamiento empírico con un macrólido en el 68,5% de los casos. La necesidad de ingreso hospitalario fue inversamente proporcional a la edad del paciente.
ConclusionesSegún este estudio, los niños con NAC de mayor edad tuvieron la mayor proporción de infección por MP, siendo poco sintomáticos y con escasa elevación de parámetros inflamatorios, pudiéndose beneficiar del tratamiento empírico con macrólidos. Por consiguiente, conocer la epidemiología de un área geográfica podría ser importante para el abordaje de las NAC en niños.
Community-acquired pneumonia (CAP) in children is associated with significant morbidity in developed countries and a high mortality in developing countries, with an annual incidence of 30 to 40 cases per 1000 children aged less than 5 years and an annual incidence of hospitalization of 15.7 cases per 10000 children under 18 years.1
The inclusion in the routine immunization schedule of the Haemophilus influenzae type b vaccine and later the pneumococcal conjugate vaccine has caused a shift in the epidemiology of paediatric CAP. The importance of Mycoplasma pneumoniae (MP) as the aetiological agent has increased, especially in children aged 5 years or older.2
Although several clinical rules have been proposed, there are no signs or symptoms with a good enough predictive value for the aetiological diagnosis of pneumonia.3 The introduction of polymerase chain reaction (PCR) for diagnosis of MP has led to a breakthrough in its management, as it offers quick results with a high sensitivity and specificity that can guide treatment.4
The aim of our study was to describe the epidemiology, clinical manifestations and treatment of community-acquired MP pneumonia (CAMP) in a paediatric population over a period of 6 years.
Materials and methodsThe sample consisted of patients aged less than 14 years who underwent microbiological testing for MP (PCR for detection of MP in nasopharyngeal swab samples and/or serological testing for MP in the acute phase) due to clinical suspicion of CAMP at the discretion of the paediatrician in charge in an urban paediatric emergency department in Valencia (Spain) between January 1, 2010 and December 31, 2015. We performed a retrospective review of the health records and the findings of chest radiographs for these patients.
The inclusion criteria were having undergone a chest X-ray examination in the emergency department in the previous 72h or at the time of diagnosis. We excluded patients in whom the radiographic findings ruled out CAP, as determined by a paediatrician–researcher that was blinded to the patient's demographic and clinical information. Patients underwent additional microbiological studies and other diagnostic tests at the discretion of the physician. At the time of diagnosis of CAP, a sample was collected for measurement of the white blood cell (WBC) count and the serum levels of C-reactive protein (CRP) and procalcitonin.
We defined a CAMP case as a patient presenting with the following two criteria: (1) MP infection confirmed by positive PCR for MP in nasopharyngeal swab samples (Progenie Molecular RealCycler®) or detection of antibodies against MP through a MP-specific IgM assay during the acute phase of disease (ELISA IgM, Vircell®) and (2) radiographic evidence of pneumonia.5 The radiographic criteria included presence of consolidation (lung opacity with or without air bronchograms), other infiltrates (alveolar or interstitial densities) and pleural effusion. We did not consider peribronchial thickening or atelectasis criteria for pneumonia.
The exclusion criteria were relevant chronic illness (neoplasia, transplant, lung disease other than asthma, immunodeficiency or severe heart disease), recent hospitalization (within the past 7 days), residency in an extended-care facility, and previous inclusion in the study in the past 28 days.
We obtained follow-up data on the patients following the collection of the sample for MP detection from the electronic health records, which include information for all health care services received in the public health system of the province of Valencia (emergency, inpatient, primary and hospital outpatient care).
Statistical analysisWe performed a descriptive analysis, summarizing qualitative data as absolute frequencies and percentages and quantitative data using the median and interquartile range (IQR). We assessed for significant associations using the χ2 test or the Fisher exact test as appropriate for categorical variables, and analysis of variance (ANOVA), the Student t-test or the Kruskal–Wallis test as appropriate for continuous variables. The analysis was performed with the Statistical Package for the Social Science, version 22.0 for Windows (SPSS, Chicago, IL, USA). We defined statistical significance as a P-value of less than 0.05.
ResultsStudy sampleA total of 636 patients with a diagnosis of CAP underwent microbiological testing for MP. Of those, 611 (96.1%) patients met the radiographic criteria of CAP, and 25 (3.9%) were excluded because they did not fulfil the radiological criteria. The tests used for MP diagnosis were PCR alone in 558 patients (91.3%), specific IgM assay alone in 40 (6.5%) and both in 13 (2.1%).
Of the 611 cases with radiographic evidence of pneumonia, 162 (26.5%) were diagnosed as CAMP. The median age at diagnosis of CAMP was 6 years (IQR, 4–9 years), and 84 of these patients (51.9%) were female. The age ranged between 6 months and 13 years and 11 months. Of all patients with CAMP, 148 (88.3%) had positive PCR results, 19 (8.6%) had positive IgM results, and 5 (3.1%) had positive results in both tests. All the patients that underwent both tests had positive results in both, except for one child that had a positive result in the antibody assay and a negative result in the PCR test.
Table 1 shows the demographic characteristics, clinical manifestations and laboratory findings of patients with CAMP by age group. Of the 162 patients, 32 (19.8%) reported a history of asthma or reactive airway disease.
Demographic characteristics, clinical manifestations and laboratory findings for patients with CAMP by age group.
| <2y | 2–5y | 6–9y | 10–13y | Total | P | |
|---|---|---|---|---|---|---|
| n=12 (7.4%) | n=70 (43.2%) | n=46 (28.4%) | n=34 (21%) | n=162 (100%) | ||
| Demographic data | ||||||
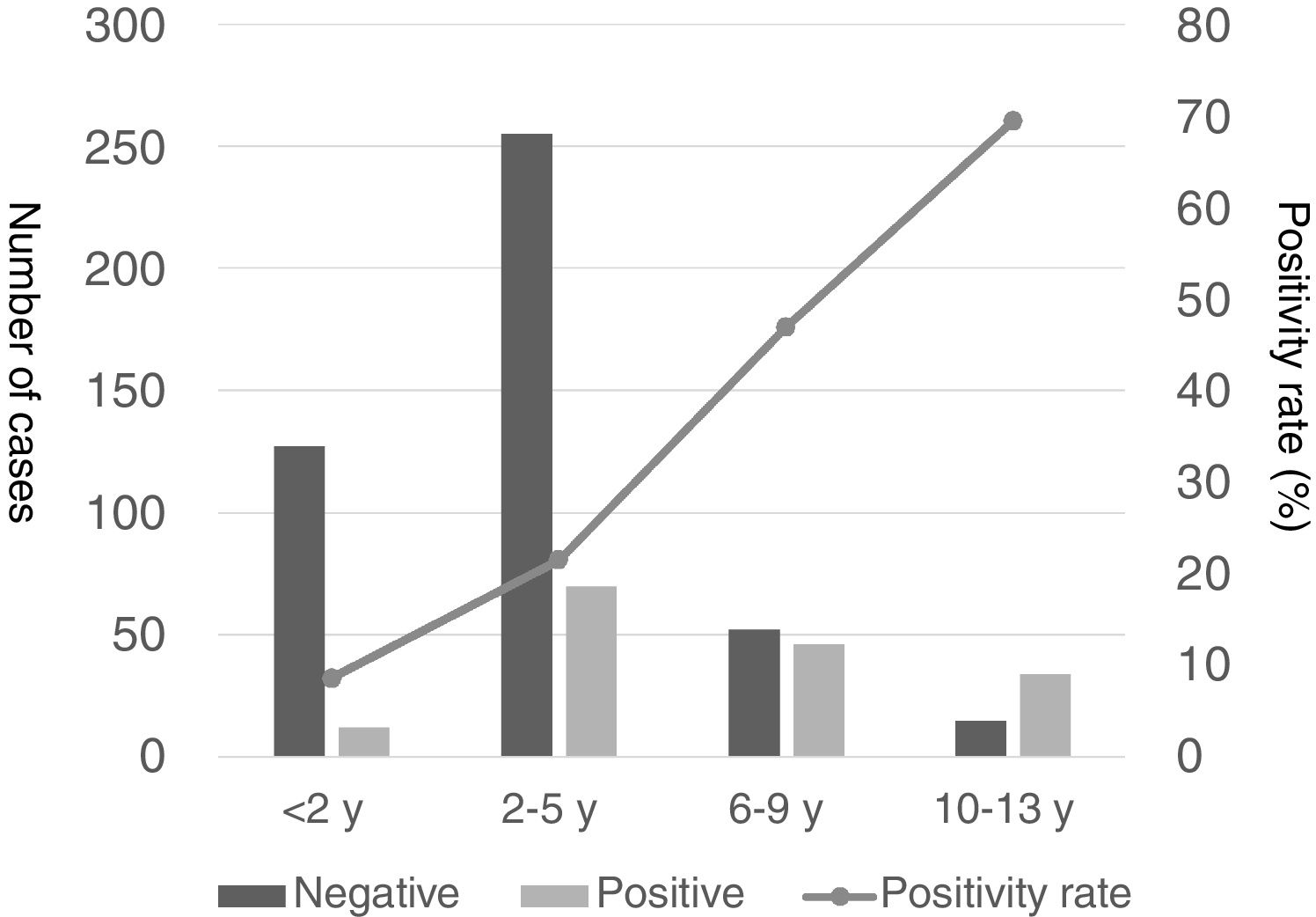
| Positivity rate | 8.6% | 21.5% | 46.9% | 69.4% | 26.5% | <.001 |
| Sex (female) | 3 (25.0%) | 35 (41.7%) | 27 (32.1%) | 19 (55.9) | 84 (51.9%) | NS |
| Recurrent Wheezing* | 8 (66.7%) | 29 (41.4%) | 12 (26.1%) | 4 (14.8%) | 53 (32.7%) | .001 |
| Treatment | ||||||
| Empiric macrolide** | 2 (16.7%) | 17 (24.3%) | 15 (32.6%) | 10 (29.4%) | 44 (28.2%) | NS |
| Empiric β-lactam** | 8 (66.7%) | 22 (31.4%) | 12 (26.1%) | 7 (20.6%) | 49 (30.2%) | .024 |
| Empiric β-lactam+macrolide | 1 (8.3%) | 30 (42.9%) | 19 (41.3%) | 17 (50.0%) | 67 (41.4%) | NS |
| Systemic corticosteroids | 4 (33.3%) | 12 (17.1%) | 1 (2.2%) | 1 (2.9%) | 18 (11.1%) | .002 |
| Oxygen therapy | 3 (25.0%) | 17 (24.3%) | 2 (4.3%) | 1 (2.9%) | 23 (14.2%) | .003 |
| Admission | 9 (75.0%) | 35 (50.7%) | 13 (20.3%) | 7 (10.9%) | 64 (39.8%) | .001 |
| Length of stay | 5 (3–6.7) | 3 (1.5–5.0) | 3 (2.0–3.5) | 2 (1.0–4.0) | 3 (2.0–5.0) | NS |
| Manifestations | ||||||
| Pleural effusion | 1 (8.3%) | 11 (15.7%) | 7 (15.2%) | 3 (8.8%) | 22 (13.6%) | NS |
| Fever (°C) | 38.5 (38.0–39.0) | 38.9 (38.5–39.2) | 39 (38.4–39.5) | 39.5 (39–39.7) | 39 (38.5–39.5) | .001 |
| Duration of fever (hours) | 84 (33–120) | 96 (39–144) | 96 (48–144) | 144 (96–168) | 96 (48–144) | .01 |
| Laboratory | ||||||
| Leucocyte count (cells/μL) | 11550 (9500–16775) | 11200 (9525–13950) | 9450 (7650–12250) | 8300 (7000–9600) | 10200 (8000–12900) | .005 |
| Neutrophil count (cells/μL) | 6200 (2600–10150) | 7900 (5225–11725) | 6150 (4500–9700) | 5600 (4600–7000) | 6400 (4850–9950) | NS |
| Lymphocyte count (cells/μL) | 4800 (3625–5125) | 2300 (1625–3200) | 1850 (1500–2100) | 1500 (1100–1900) | 2000 (1450–2950) | <.001 |
| CRP (mg/dL) | 2.1 (1.3–3.9) | 3.4 (1.5–6.1) | 2.9 (1.6–6.1) | 3.2 (1.9–7) | 3 (1.5–6.0) | NS |
| PCT (ng/mL) | 0.7 (0.1–1.4) | 0.1 (0.1–0.1) | 0.1 (0.1–0.4) | 1.2 (0.0–2.4) | 0.1 (0.1–0.4) | NS |
CRP, C-reactive protein; NS, not significant; PCT, procalcitonin. Statistically significant differences are presented in boldface. Data expressed as absolute frequency and percentage or median and interquartile range.
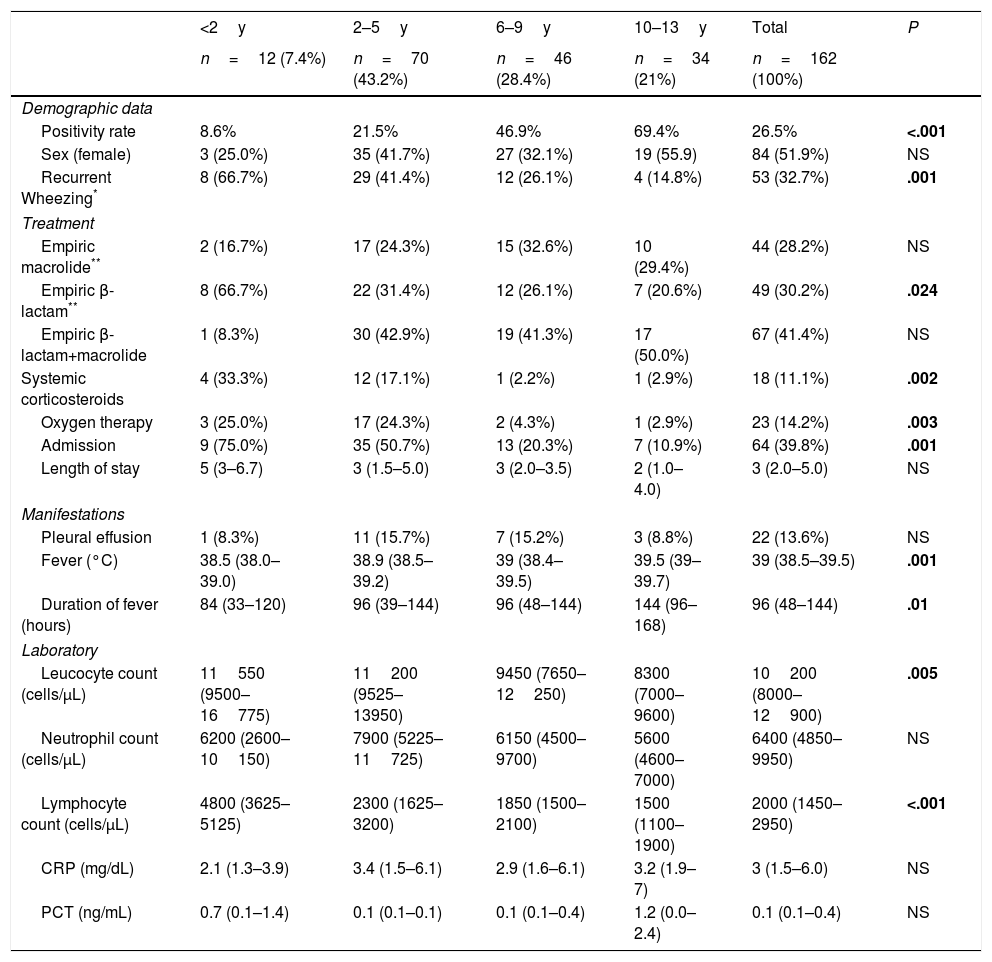
The proportion of patients with CAP that tested positive for MP increased progressively with age, with positive results in 69.4% of children aged more than 9 years compared to 8.6% in children aged less than 2 years, 21.5% in children aged 2–5 years and 46.9% in those aged 6–9 years (Fig. 1).
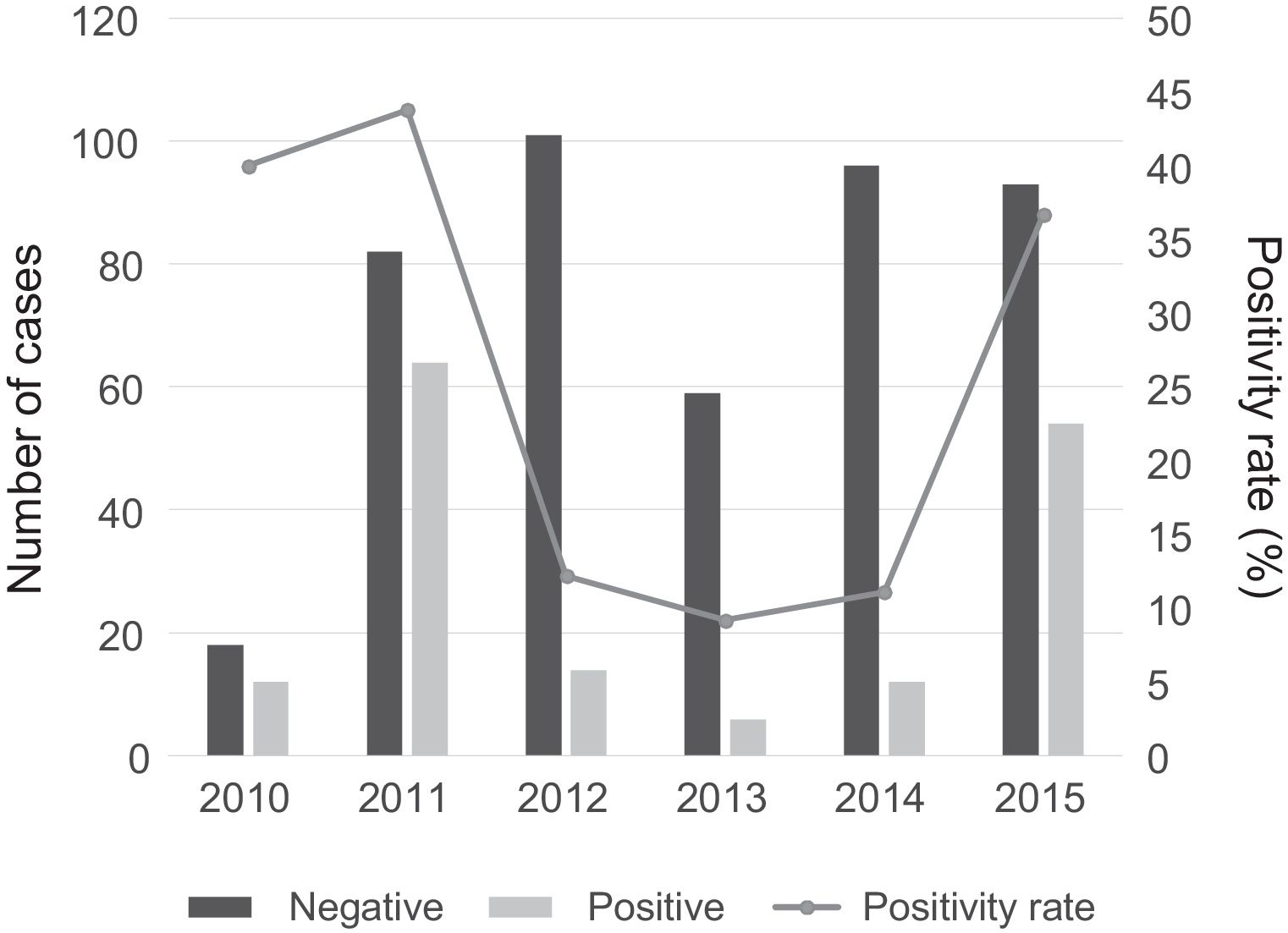
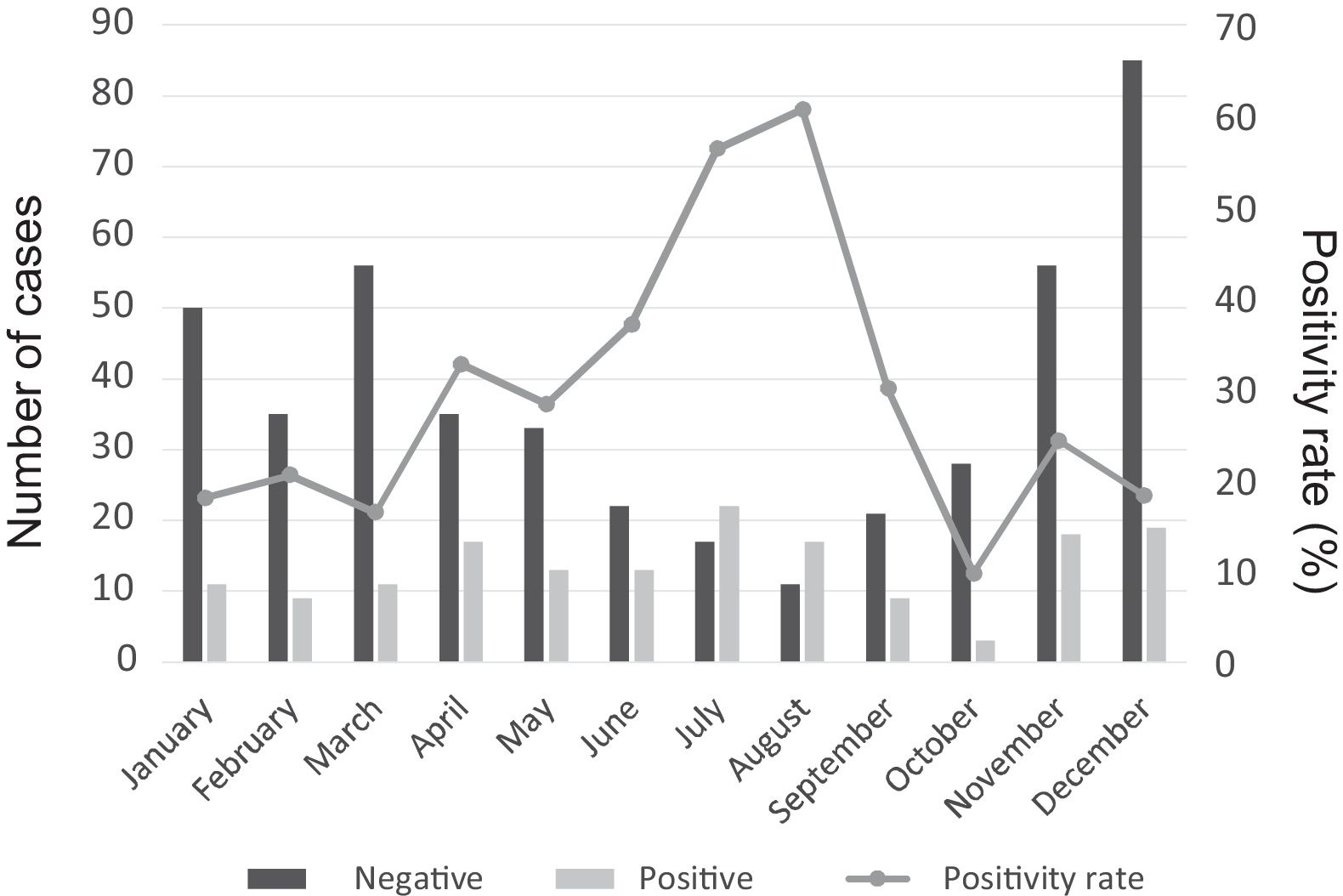
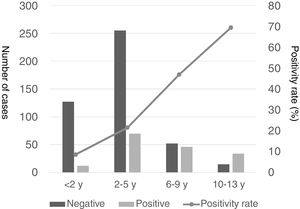
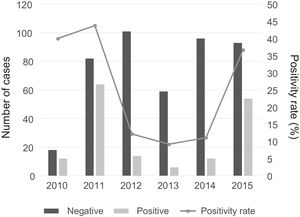
There was a peak of cases in 2011 and later in 2015, with 4- to 10-fold increases in incidence compared to other years (Fig. 2). The seasonal distribution of patients with CAMP showed higher positivity rates in summer compared to other seasons (49.5% vs. 18% in winter, 32.3% in spring and 19.1% in autumn; P<.05), with statistically significant differences in summer (OR=4.5; 95% CI, 2.6–7.8; P<.001) and spring (OR=2.2; 95% CI, 1.3–3.7; P=.004) compared to winter. Fig. 3 shows the distribution of cases by months of the year.
We found follow-up data for 154 of the 162 patients (95.1%): 120 (74.1%) followed up by a primary care paediatrician, 51 (31.5%) in outpatient care in our hospital, and 96 (59.3%) in inpatient care. Several patients were followed up in more than one setting.
Other pathogensTesting for other pathogens was done in 116 of the patients with CAMP (71.6%). Nasopharyngeal swab samples were collected to test for respiratory viruses in 30 of the 162 patients (18.5%) and for Chlamydophila pneumoniae in 90 (55.6%), blood samples collected for culture in 21 patients (13.0%) and for serological testing for respiratory viruses during the acute phase of disease in 10 (6.2%), and pleural fluid samples collected for culture in 4 patients (2.5%).
We found evidence of coinfection of MP and respiratory viruses in 7 of the 30 patients that had undergone testing for these viruses in nasopharyngeal swab samples (23.3%) and coinfection with C. pneumoniae in 1 out of 90 patients tested for this bacteria (1.1%). Three different viruses were detected in 2 children, and two different viruses in another 2 children. A single virus was detected in the remaining patients with viral coinfection. The virus detected most frequently was respiratory syncytial virus (4 cases), followed by parainfluenza and influenza viruses (each in 2 patients). Other detected viruses were adenovirus, rhinovirus, metapneumovirus, bocavirus and coronavirus (in 1 patient each). There were no cases of detection of pathogens in blood or pleural fluid.
Clinical presentationThe most frequent presenting symptom in patients with CAMP was cough (92.6%), followed by fever (85.8%), rhinorrhoea (45.1%), vomiting (26.5%), decreased appetite (19.8%), rash or other cutaneous manifestation (9.9%), abdominal pain (5.6%), sore throat (5.6%), diarrhoea (4.9%), headache (4.3%) and chest pain (2.5%). The type of rash was hives in 9 patients, macular rash in 2, papular rash in 1 and macular-petechial rash in 1. The other patients with a cutaneous manifestation were evaluated by a dermatologist and received diagnoses of erythema multiforme, serum sickness-like reaction and pityriasis lichenoides et varioliformis acuta.
When we compared manifestations by age group, we only found differences in headache, which was more frequent in school-aged children (10.9% vs. 5.9% in preadolescents and no cases in the rest of the cohort; P=.034), probably due to the difficulty of assessing for it in younger children. Body temperature and duration of fever increased with increasing age (Table 1).
The most common lung sound documented during the initial evaluation in the emergency room was crackles, found in 104 of the 162 patients (64.2%), followed by decreased breath sounds in 82 (50.6%), wheezing in 33 (20.4%) and rhonchi in 6 (3.7%), with no differences between age groups. The findings of lung auscultation were normal in 22 patients (13.6%).
Of the 162 patients, 53 (32.7%) had wheezing requiring treatment with bronchodilators, with a higher incidence in younger children and hospitalized patients (48.4% hospitalized vs. 22.4% not hospitalized; P=.001). Out of the 53 patients with wheezing, 18 (34%) required systemic corticosteroid therapy, with the same distribution by age.
The most frequent radiological pattern was segmental infiltration (62.3%). Other, less frequent patterns were interstitial (27.2%) and lobar (13.0%). Lung consolidation was round in 7 cases (4.3%). Fifteen patients (9.3%) had multilobar involvement; 79 (48.8%) patients had associated perihilar thickening and 5 (3.1%) had atelectasis. There was evidence of pleural effusion in 22 patients (13.6%). The effusion was small in all cases (≤12mm), except in one case where it measured 28mm. Patients with effusion only differed from patients without effusion in the rate of hospitalization (44.4% vs. 67.6%; P=.052) and a less frequent empiric use of macrolides (48.6% vs. 18.2%; P=.008). There were no significant differences in the radiological features between age groups.
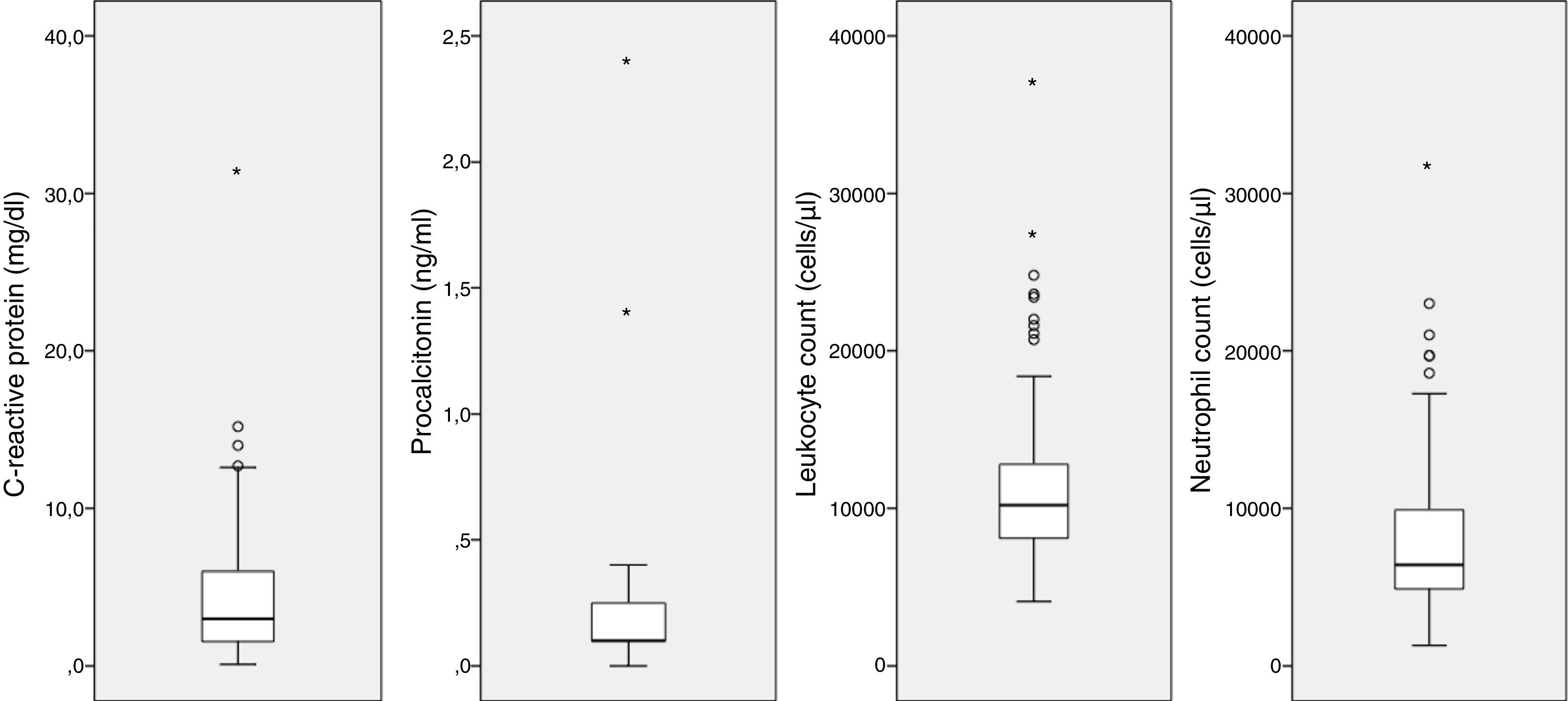
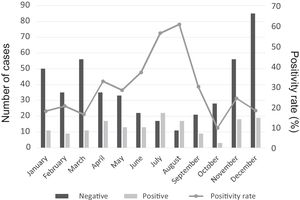
Laboratory findingsA WBC count was performed in 105 of the 162 patients with CAMP (64.8%), the serum level of CRP was measured in 104 (64.2%) and the level of procalcitonin in 11 (0.7%). Fig. 4 provides a graphic representation of the blood test results. We ought to mention the low elevation of inflammatory markers, with the following median values: leukocytes, 10200cells/μL (IQR, 8000–12900); neutrophils, 6400cells/μL (IQR, 4850–9950); CRP, 3mg/dL (IQR, 1.5–6); and procalcitonin, 0.1ng/mL (IQR, 0.1–0.4).
The WBC and lymphocyte counts were significantly higher in younger children, which could be due to the different range of normal in this age group, while we found no differences in the neutrophil count or the CRP or procalcitonin levels (Table 1). We found no significant differences in the median values of acute phase reactants when we compared patients based on the presence of pleural effusion or the need for hospital admission.
TreatmentEmpirical treatment with macrolides before the results of testing for MP became available was more frequent in older patients (79.4% in the 10–13 years age group vs. 25% in the <2 years age group; P=.001), with a predominance of clarithromycin (59.5%) over azithromycin in patients that received macrolide therapy. When we divided the study follow-up in two periods, we found that azithromycin was used more frequently in the second period (2013–2015) compared to the first (2010–2012): 91.5% vs. 3.1% (P<.001).
Before the visit when the sample for testing for MP was collected, 76 patients (46.9%) received prescriptions for antibiotic treatment: 72 (44.4%) for a beta-lactam antibiotic, 3 (1.9%) for a macrolide, and 1 for a beta-lactam antibiotic combined with a macrolide (in all, the macrolide was clarithromycin). Thirty-one patients (42.5%) had received a diagnosis of pneumonia previously in the episode. We did not find a higher proportion of empiric macrolide treatment prescribed at the time of collection of the sample for testing for MP in patients that were receiving a beta-lactam antibiotic in monotherapy or in patients that had received a diagnosis of pneumonia in the past few days.
Empiric treatment was modified when the test results became available in 68 of the 162 patients (42%). The change consisted in switching from a beta-lactam antibiotic to a macrolide in 21 of these 68 patients (30.9%), switching from combined treatment with a beta-lactam antibiotic and a macrolide to monotherapy with the macrolide in 26 (38.2%), and addition of a macrolide to ongoing treatment with a beta-lactam antibiotic in 21 (31%). Seven (4.3%) patients were treated with a beta-lactam antibiotic in monotherapy without a macrolide, which reportedly achieved resolution of symptoms.
Sixty-four (39.8%) patients were admitted to hospital. The rate of hospitalization was higher in children aged less than 2 years. Twenty-three (14.2%) patients needed supplemental oxygen, with a higher proportion of oxygen therapy in younger children. Only 3 children required admission to an intermediate care unit, and none required mechanical ventilation. None of the patients required admission to the intensive care unit. All patients achieved complete clinical and radiological resolution. Only one of patients with pleural effusion (4.5%) required drainage.
DiscussionTo date, many studies have described the clinical manifestations of CAMP in hospitalized children,6–13 but as far as we know, this is one of the largest studies describing the characteristics of CAMP in children, including outpatients. Despite its retrospective nature, the high proportion of patients for which we could obtain follow-up data allowed us to establish the course of this infection in a representative sample from the general population, as opposed to only patients that required admission.
We found two incidence peaks in the period under study: the first one in 2011, which had already been described in other populations in Europe,14–16 and the second in 2015. This is consistent with the cyclic epidemic pattern of CAMP, with outbreaks known to occur every 4–7 years. The reason for this is not fully understood yet. Outbreaks may be facilitated by the waning of herd immunity and shifts in the MP serotypes circulating in the human population.4
The seasonal prevalence differs depending on the population under study. In countries with temperate climates, outbreaks of MP infection tend to occur in the summer or early autumn, when other respiratory infectious pathogens are less prevalent.9 In our sample, we found a higher proportion of positive cases in summer. However, there were incidence peaks in July, November and December. A higher incidence of respiratory tract infections in November and December combined with a smaller proportion of MP infections could explain the higher number of cases of pneumonia but with a lower positivity rate for MP in these months.
Although theoretically MP is a pathogen that predominantly infects school-aged children, we found the largest number of cases in the 2-to-5-years age group. Most studies in the literature have reported peaks in the 5-to-9-years age group.8,9,12,17 The positivity rate in children with lower respiratory tract infection and its distribution by age was similar to those reported in a recently published single centre study conducted in Denmark.18 A multicentre, population-based study of community-acquired pneumonia in children in the United States found that the distribution of cases of MP was fairly across age groups, although its proportion out of the total cases of pneumonia steadily increased with age.2 This is similar to what we found in our sample, with a higher positivity rate in older patients. In light of these results, physicians may consider empiric treatment of CAP with a macrolide in older children and reserving testing for detection of MP for younger children, since detection of MP may result in treatment changes.
Although current guidelines recommend PCR and single-sample serological testing to diagnose MP infections,19,20 the gold standard for diagnosis remains a fourfold increase in the antibody titre measured in paired sera.21 There are drawbacks to this approach in clinical practice, as it may hinder decision-making regarding treatment initiation and may be only useful as a retrospective confirmatory test. The development of PCR has been a breakthrough in this regard. The data in our study showed a clear shift from the use of serological methods to the use of PCR for diagnosis. However, some concerns remain unresolved, such as the presence of asymptomatic carriers, previously described in children, which complicates the interpretation of PCR results.22
We also found a changing trend in the use of clarithromycin, which was displaced by azithromycin in the second period (91.5% vs. 3.1% of azithromycin out of all macrolide prescriptions), a treatment that has a more convenient dosing schedule. In fact, the 2011 United States guidelines recommended azithromycin as the antibiotic of choice.19
The laboratory characteristics of CAMP were remarkable. We ought to highlight the low elevation of CRP, with levels that were very similar to those reported in other studies.9,17,23 The main concern about treating a child with CAMP with a macrolide in monotherapy is the possibility of co-infection with S. pneumoniae and the drug resistance associated with this pathogen. An article published by Chiu et al. reported higher levels of CRP in cases of MP with S. pneumoniae coinfection compared to MP monoinfection (66.5±24.0mg/L vs. 296.1±114.3mg/L; P<.01).12 In the sample under study, there was only 1 detected case of bacterial coinfection (C. pneumoniae). Other authors have reported proportions of bacterial coinfection of up to 2% in patients with CAMP.24 This difference could be explained by several reasons: local epidemiology influenced by vaccination coverage, the setting of the sample under study, the kind of samples used for testing or the microbiological criteria used in the case definition.
We found differences in the clinical presentation between age groups. Wheezing associated with CAMP was significantly more common in younger children, although there are limitations to our study due to the low frequency of testing for viral coinfections. Nevertheless, the stratified analysis that included the patients in whom viral detection tests had been performed in nasopharyngeal swab samples found a higher prevalence of wheezing in patients with viral coinfection, although the difference was not statistically significant (71.4% vs. 56.5%; P=.481). The highest fevers corresponded to older children, in whom the diagnosis occurred after a longer time had elapsed from onset of fever. Of all patients with CAMP, 14.2% were afebrile at the time of diagnosis. This is similar to the findings of a study of 257 episodes of pneumococcal pneumonia in children, of whom 10% presented without fever.25
Unlike other studies that found that diarrhoea, vomiting and upper respiratory tract involvement were more common in younger children,9,17 we did not find differences in these variables. Cutaneous manifestations develop in up to 25% of MP infections.4 In our study, 1 out of every 10 patients with CAMP presented with a rash.
Some studies have found evidence of different manifestations in children with CAMP and viral coinfection.12,13 In our sample, the analysis of patients in whom testing was performed for viral detection in nasopharyngeal swab samples found that, compared with patients with negative results, patients with viral coinfection tended to be younger (median age 2.6 vs. 5.1 years; P=.033) and were more likely to be hospitalized (100% vs. 60.9%; P=.048).
The need of treatment in CAMP is still under debate.26,27 In our sample, most patients received a macrolide before or after the results of testing for detection of MP became available. A previous Cochrane review21 concluded that there is insufficient evidence about the efficacy of antibiotics in the treatment of MP-related lower respiratory tract infections in children.
There are some limitations to our study. First of all, a positive PCR or IgM test does not rule out the possibility of a past infection or the presence of coinfection. This is a common limitation of similar studies.7–12 However, this allowed us to describe the management of these infections in clinical practice with the tests available for use in the acute phase, and the follow-up and treatment chosen based on the results of these tests. Secondly, the retrospective nature of the study precluded the collection of samples for assessment of other microbial coinfection or for follow-up in all patients. Finally, the lack of routine testing for mutations that confer macrolide resistance prevented us from establishing the rate of drug resistance in our sample. It is well known that there has been a worldwide increase in the circulation of macrolide-resistant MP strains.28–30
In conclusion, according to this study, among the children with CAP, the proportion of infection by MP is highest in those who are older, have milder symptoms and have lower levels of inflammatory markers, so these subsets could benefit from empiric treatment with a macrolide. Thus, knowledge of the epidemiology in a given geographical area may be important for the management of CAP in children.
We thank Martin J. Smyth, B.A., for his help in revising the English draft of this article.
Please cite this article as: Aguilera-Alonso D, López Ruiz R, Centeno Rubiano J, Morell García M, Valero García I, Ocete Mochón MD, et al. Características clínicas y epidemiológicas de las neumonías adquiridas en la comunidad por Mycoplasma pneumoniae en una población española, 2010–2015. An Pediatr (Barc). 2019;91:21–29.
Previous presentations: This study was presented at the 35th Annual Meeting of the European Society for Paediatric Infectious Diseases, April 2017, Madrid, Spain; and at the VIII Congress of the Sociedad Española de Infectología Pediátrica, March 2016, Valencia, Spain.