Oral feeding of preterm newborns (PTNB) is hampered by their immaturity and intercurrent diseases, which can prolong their hospital stay. The objective of this study was to assess the effectiveness of a program that combines tactile, kinesthetic and oral stimulation (T + K + OS) compared to another intervention based on exclusively oral stimulation (OS), in the time necessary to achieve independent feeding and hospital discharge.
Patients and methodsA clinical study of 2 randomized groups (OS vs. T + K + OS) was carried out on 42 PTNB with gestational age between 27-32 weeks and birth weight > 900 g. The stimulation programs were carried out in sessions of 15 min, for 10 days.
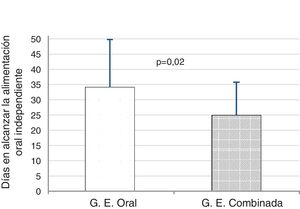
ResultsThe PTNBs in the T + K + OS group achieved independent oral feeding earlier, compared to the OS group (24.9 ± 10.1 vs. 34.1 ± 15.6 days, P = .02). An analysis of covariance was performed, which confirmed that the birth weight and gestational age covariates had significant effects on time to reach suction feeding (birth weight: F[1, 38] = 5.79; P = .021; gestational age: F[1, 38] = 14.12; P = .001) and that once its effect was controlled, the intervention continued to have a significant effect (F[1, 38] = 6.07; P = .018). The T + K + OS group, compared to the OS group, achieved an earlier hospital discharge (39 ± 15 vs. 45 ± 18 days), although the differences were not significant (P = .21).
ConclusionsCombined therapies that include T + K + OS are more effective than OS alone, in order to achieve independent oral feeding in PTNBs.
La alimentación oral de los recién nacidos pretérmino (RNPT) está dificultada por su inmadurez y enfermedades intercurrentes, lo que puede prolongar su estancia hospitalaria. El objetivo de este estudio fue valorar la efectividad de un programa que combina la estimulación táctil, kinestésica y oral (ET + K + O) frente a otro de estimulación oral sola (EO), en el tiempo necesario para lograr la alimentación independiente y el alta hospitalaria.
Paciente y métodosEstudio clínico de 2 grupos aleatorizados (EO vs. ET + K + O), realizado en 42 RNPT con una edad gestacional entre 27-32 semanas y un peso al nacimiento > 900 g. Los programas de estimulación fueron realizados en sesiones de 15 min, durante 10 días.
ResultadosLos RNPT del grupo de ET + K + O consiguieron antes la alimentación oral independiente, en comparación con el grupo de EO (24,9 ± 10,1 vs. 34,1 ± 15,6 días, p = 0,02). Se realizó un análisis de covarianza, observando que las covariables PN y EG tuvieron efectos significativos en el tiempo hasta alcanzar la alimentación por succión (peso al nacimiento: F[1, 38] = 5,79; p = 0,021; edad gestacional: F[1, 38] = 14,12; p = 0,001) y que una vez controlado su efecto, la intervención seguía teniendo un efecto significativo (F[1, 38] = 6,07; p = 0,018). El grupo de ET + K + O, en comparación con el de EO, consiguió antes el alta hospitalaria (39 ± 15 vs. 45 ± 18 días), si bien la diferencia no fue significativa (p = 0,21).
ConclusionesLas terapias combinadas que asocian ET + K + O son más eficaces que la EO sola, para lograr la alimentación oral independiente en los RNPT.
In recent decades, there has been an increase in the number of infants born preterm (PT) that required prolonged hospitalization and specialised care.1 Many of these infants have difficulties to initiate oral feeding, which hinders breastfeeding. Oral feeding requires coordination of sucking, swallowing and breathing, which is present from birth in infants born to term.2,3 Most PT infants (born before 34 weeks of gestation) have not developed this ability at the time of birth.4
Some authors consider oral feeding the earliest indicator of wellness in newborn (NB) development.5 The development of effective feeding depends on multiple factors, such as gestational age, muscle tone, the maturity of the nervous and gastrointestinal systems and the presence of comorbidities, especially those involving the respiratory or gastrointestinal systems.6 The feeding skills of PTNBs may also be affected by the level of behavioural development, environmental conditions in the neonatal intensive care unit (NICU) and hypotonia in the infant.7–9 On the other hand, feeding difficulties may delay hospital discharge, affect parent-child interactions and result in increased costs for the health care system.10–12
Uncoordinated sucking patterns in the PTNBs may signal the presence of neurologic impairment and delayed motor skill development,13–15 so it is important to identify these infants as soon as possible to offer early intervention. Greene et al16 highlighted the need for early sensorimotor intervention in PTNBs to minimise the impact of the early negative sensory experiences in the NICU, which may alter development and behaviour and delay the transition to independent oral feeding.
Broadly speaking, sensorimotor interventions aimed at improving feeding skills in PTNBs have been developed to decrease oral hypersensitivity, improve the mobility and strength of the muscles involved in sucking and swallowing and improve sensorimotor processes in breathing.17 At present different strategies are being used to promote the development of oral feeding skills in PT infants. They include oral motor interventions,18 oral stimulation with non-nutritive sucking,19 non-oral sensorimotor stimulation, such as auditory, tactile, visual or vestibular stimulation,20 or tactile/kinaesthetic stimulation, alone21 or combined with massage therapy at moderate pressure.22,23
Two systematic reviews on the effect of oral stimulation (OS) in PTNBs concluded that interventions with OS can shorten the duration of parenteral nutrition, the transition to oral feeding and the length of stay.17,18 On the other hand, some studies have found that multisensory interventions improve feeding behaviour in PT infants, reducing the time to transition from enteral tube feeding to full oral feeding,20,24 but few studies have compared the effects of OS versus combined interventions. For this reason, we conducted a study with the aim of assessing the effectiveness of a combined intervention with tactile, kinaesthetic and oral stimulation (T + K + OS) compared to OS exclusively in PT infants based on the time elapsed to achieving independent oral feeding and the length of stay.
Patients and methodsStudy sample and designWe conducted a prospective, experimental parallel-group study with random allocation to the groups. The researcher that analysed the data was blinded to the intervention delivered in each group. The study was conducted in PTNBs admitted to the Department of Neonatology of our hospital in 2016. We obtained a convenience sample by enrolling all infants admitted that year that met the inclusion criteria and none of the exclusion criteria.
The inclusion criteria were: birth at 27 to 32 weeks of gestation, birth weight of 900 g or greater, weight adequate for gestational age, nasogastric or orogastric tube feeding, haemodynamic and clinical stability, absence of nutritive sucking and informed consent of parents to participation in the study. We excluded PTNBs with congenital orofacial anomalies, intraventricular haemorrhage grade iii or iv, post-haemorrhagic hydrocephalus, periventricular leukomalacia, severe systemic disease (such as sepsis or necrotising enterocolitis), major surgery and invasive mechanical ventilation with endotracheal intubation during the intervention.
The study was approved by the Clinical Research Ethics Committee of the Hospital Universitario de Canarias. We obtained informed consent for every participant before starting any of the study procedures.
Study protocolThe intervention was delivered to all patients by a single provider, a physical therapist of the paediatric unit of the Department of Rehabilitation in our hospital, when patients had a postmenstrual age of 32 to 33 weeks, when most infants have not yet achieved full suck-swallow-breathe synchrony.
Stimulation was delivered when infants were in the quiet-alert state.25 The intervention took place half hour before the feed to minimise the risk of aspiration. Infants in both groups received stimulation once a day for 15 minutes for a period of 10 days.
Oral stimulation group (OS). Infants received perioral and intraoral stimulation (cheek, jaw and tongue movement) following the protocol described by Fucile et al,26 modified as follows: in the last manoeuvre, we used the little finger instead of a pacifier/dummy to stimulate sucking and to keep the use of the pacifier from interfering with initiation of sucking at the breast.27
Tactile, kinaesthetic and oral stimulation group (T + K+OS). The intervention consisted in tactile and kinaesthetic stimulation, and the OS intervention we have just described, on alternate days. Tactile stimulation consisted of stroking at moderate pressure22 and manipulation manoeuvres with a gentle, gradual, rhythmic, continuous and firm quality, for 5 minutes a day, keeping both hands of the physical therapist in contact with the skin of the infant at all times.28 In order to increase the flexor tone, the manoeuvres led to the flexion of the head, neck and trunk and brought the upper extremities and lower extremities over to the torso, keeping the infant aligned along the midline. Kinaesthetic stimulation consisted of gentle, passive movements of the joints of the upper and lower extremities in a cephalocaudal and proximodistal direction for 10 minutes. The infant was placed in the decubitus position.
The stimulation regimen was postponed for a few hours in infants of either group in the following cases: if the infant had experienced episodes of apnoea, bradycardia or oxygen desaturation in the 2 hours prior to the scheduled intervention, if the nursing staff considered postponement appropriate due to clinical worsening or because the infant had undergo tests that required rest (for example, a previous eye examination); and infants that had exhibited signs of stress and disorganization during stimulation, such as hiccups, choking, changes in colour, pauses in breathing or laboured breathing, tremors or cough. All infants underwent the 10 sessions scheduled in the intervention.
All admitted PTNBs had kangaroo care sessions with their parents as part of the routine care protocol in the neonatal unit of the neonatal of the Complejo Hospitalario Universitario de Canarias. Initiation and progression of oral feeding adhered to the protocol of the neonatal unit and was similar in both groups.
Below is a summary of the nutrition protocol for PT infants:
Weight greater than 1500 g and/or 32-34 weeks:
- none-
Enteral nutrition. If an adequate progression of enteral nutrition could not be achieved by 72 hours post birth, initiation of parenteral nutrition.
- none-
Weight greater than 1800 g and 32-34 weeks: oral nutrition with sucking based on sucking efficiency and ability to coordinate sucking, swallowing and breathing. The infant started with non-nutritive sucking and progressed towards full, independent oral feeding.
- none-
Gold standard: breastfeeding, without fortification through 7-14 days (> 100 mL/kg/day) and fortified thereafter.
- none-
Mothers were provided with a breast pump and instructed on how to preserve and store breastmilk and how to transport it from home to hospital.
- none-
Until the infant can suck directly at the breast, delivery of expressed maternal milk through a nasogastric or orogastric tube or a bottle.
- none-
Once the infant is capable of breastfeeding, the mother is taught the correct positioning of the infant at the breast and the signs of a good latch.
Weight between 1000-1500 g:
- none-
Initiation of parenteral nutrition and trophic feeding on day 1.
- none-
If the infant exhibits adequate oral tolerance, increase by 10-30 mL/kg/day until reaching 150-180 mL/kg/day or the necessary volume to achieve a weight gain of 15-20 g/kg/day.
Weight less than 1000 g:
- none-
Initiate parenteral nutrition on day 1. Wait at least 24 hours to initiate trophic feeding.
- none-
If the infant exhibits adequate oral tolerance, between days 2 and 7, increase by 10-20 mL/kg/day, until reaching 150-180 mL/kg/day or the necessary volume to achieve a weight gain of 15-20 g/kg/day.
- none-
Length of stay.
- none-
Postnatal age (days) at which the infant achieves full oral feeding with independent sucking, defined as the number of days elapsed from birth to the infant being able to take all feeds in a day through independent sucking or completion of 8 successful oral feeds in 2 consecutive days.26
- none-
Days elapsed to full oral feeding through independent sucking from initiation of the intervention.
- none-
Days of combined tube and oral feeding.
- none-
Weight at the end of the intervention.
- none-
Weight at the time independent oral feeding is achieved.
- none-
Number of feeds at the breast at the end of the intervention.
- none-
Type of feeding at discharge. We defined exclusive breastfeeding as the infant feeding exclusively on human milk (sucking at the breast or expressed human milk), with possible oral supplementation with vitamins, minerals or medication, mixed feeding when the infant received maternal milk and formula, and formula feeding when the infant was fed only formula.
We have expressed categorical data as absolute frequencies and percentages. We summarised quantitative data using the mean and standard deviation (SD). We compared means in the 2 groups with the t test for independent samples. We compared proportions in the 2 groups using the chi square test. We also performed an analysis of covariance to assess whether the effect of the intervention in the number of days elapsed to full independent oral feeding was maintained after controlling for covariates such as gestational age and birth weight. We considered p-values of less than 0.05 statistically significant. The statistical analysis was performed with the software SPSS version 170 (SPSS Inc; Chicago, IL, USA).
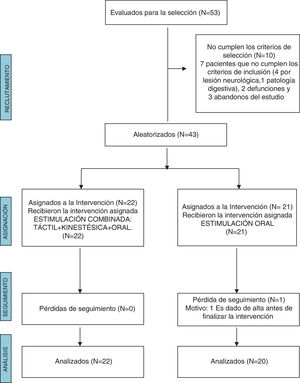
ResultsWe excluded 10 of the 53 patients evaluated for the study because they did not meet the inclusion criteria. In the OS group, 1 patient did not complete the follow-up due to being discharged before the end of the intervention. The final sample included 42 NBs, 22 in the T + K + OS group and 20 in the OS group (Fig. 1).
We did not find statistically significant differences between groups in the baseline clinical characteristics (Table 1). Table 2 presents the characteristics of the patients at the end of the intervention (gestational age, postnatal age and weight).
Baseline clinical characteristics of the 2 groups at the start of the intervention.
| Combined tactile, kinaesthetic and oral stimulation (n = 22) | Oral stimulation (n = 20) | P | |
|---|---|---|---|
| Gestational age (weeks), mean ± SD | 30.2 ± 1.5 | 29.9 ± 1.3 | .492 |
| Birth weight (g), mean ± SD | 1424.0 ± 263.5 | 1329.3 ± 318.0 | .298 |
| 1-min Apgar, mean ± SD | 7.5 ± 2.2 | 7.7 ± 1.6 | .685 |
| 5-min Apgar, mean ± SD | 8.3 ± 1.9 | 8.6 ± 0.7 | .481 |
| Sex, n (%) | |||
| Male | 12 (54.5%) | 14 (70.0%) | |
| Female | 10 (45.5%) | 6 (30.0%) | .303 |
| Multiple gestation, n (%) | 10 (45.5%) | 8 (40.0%) | .721 |
| Antenatal steroids, n (%) | |||
| No | 3 (13.6%) | 7 (35.0%) | .248 |
| 2 doses | 13 (59.0%) | 8 (40.0%) | |
| 1 dose | 6 (27.2%) | 5 (25.0%) | |
| Hyaline membrane disease, n (%) | 7 (31.8%) | 8 (40.0%) | .580 |
| Intraventricular haemorrhage, n (%) | |||
| Grade I | 3 (13.6%) | 2 (10.0%) | .757 |
| Grade II | 1 (4.5%) | 2 (10.0%) | |
| Total parenteral nutrition (days), mean ± SD | 1.0 ± 1.7 | 1.6 ± 1.9 | .291 |
| Partial parenteral nutrition (days), mean ± SD | 6.3 ± 4.7 | 8.1 ± 7.6 | .363 |
| PMA at start of full enteral nutrition (weeks), mean ± SD | 31.3 ± 1.2 | 31.3 ± 1.1 | .972 |
| Days of mechanical ventilation, mean ± SD | 5.2 ± 5.3 | 7.5 ± 11.9 | .435 |
| Days of oxygen therapy, mean ± SD | 3.1 ± 6.2 | 5.1 ± 9.8 | .416 |
| Bronchopulmonary dysplasiaa, n (%) | 2 (9.1%) | 3 (15.0%) | .555 |
| PMA at start of intervention (weeks), mean ± SD | 32.2 ± 0.3 | 32.2 ± 0.3 | .824 |
| Chronological age at start of intervention (days), mean ± SD | 13.9 ± 9.8 | 16.0 ± 9.1 | .491 |
| Weight at start of intervention (g), mean ± SD | 1546.7 ± 206.6 | 1429.1 ± 302.2 | .146 |
PMA, postmenstrual age; SD, standard deviation.
Differences not statistically significant.
Characteristics of the 2 groups at the end of the intervention and at discharge.
| Combined tactile, kinaesthetic and oral stimulation (n = 22) | Oral stimulation (n = 20) | P | |
|---|---|---|---|
| PMA at end of intervention (weeks), mean ± SD | 34.2 ± 0.3 | 34.1 ± 0.4 | .319 |
| Chronological age at end of intervention (days), mean ± SD | 26.4 ± 10.1 | 28.5 ± 9.4 | .505 |
| Weight at end of intervention (g), mean ± SD | 1924.7 ± 245.0 | 1840.6 ± 338.2 | .358 |
| Number of feedings at breast at end of intervention, mean ± SD | 1.3 ± 1.2 | 1.4 ± 1.7 | .857 |
| Weight at discharge (g), mean ± SD | 2286.3 ± 339.2 | 2368.5 ± 349.0 | .447 |
| Type of feeding at discharge, n (%) | |||
| Exclusive breastfeeding | 11 (50.0%) | 8 (40.0%) | .542 |
| Mixed feeding | 9 (40.9%) | 12 (60.0%) | |
| Formula feeding | 2 (9.1%) | 0 (0.0%) |
PMA, postmenstrual age; SD, standard deviation.
Differences not statistically significant.
We found that PT infants in the T + K + OS achieved full independent oral feeding 9 days before infants in OS group, a difference that was statistically significant (24.9 ± 10.1 days vs 34.1 ± 15.6 days; t = 2.28; P = .02) (Fig. 2).
To confirm the effect of the intervention on the days to full independent oral feeding and assess whether birth weight and gestational age modified this effect, we performed an analysis of covariance that confirmed that these covariates had a significant effect (birth weight: F[1, 38] = 5.79 [P = .021]; gestational age: F[1, 38] = 14.12 [P = .001]) and that, after correcting for this effect, the intervention continued to have an important and significant effect on the time to achieving feeding by sucking (F[1, 38] = 6.07; P = .018).
Preterm infants in the T + K + OS group were discharged 6 days before infants in the OS group, although this difference was not statistically significant (Table 3).
Characteristics associated with feeding and length of stay in the 2 study groups.
| Combined tactile, kinaesthetic and oral stimulation (n = 22) | Oral stimulation (n = 20) | P | |
|---|---|---|---|
| Days of combined feeding (tube + sucking), mean ± SD | 9.6 ± 8.6 | 12.0 ± 7.8 | 0.36 |
| Days to independent oral feeding from start of intervention, mean ± SD | 13.8 ± 9.5 | 19.1 ± 10.4 | 0.09 |
| Weight at start of full independent oral feeding (g), mean ± SD | 1919.3 ± 241.9 | 2015.2 ± 397.6 | 0.36 |
| Length of stay (days), mean ± SD | 39.0 ± 15.3 | 45.3 ± 17.6 | 0.21 |
SD, standard deviation.
At the time of discharge, 50% of infants in the T + K + OS group (n = 11) and 40% in the OS group (n = 8) received exclusive breastfeeding; most of them received expressed maternal milk (9 in the T + K + OS group and 6 in the OS group). The differences between groups were not statistically significant (Table 2).
DiscussionOur study, carried out on 2 groups of PTNBs delivered between 27 and 32 weeks’ gestation, demonstrated that the use of combined tactile, kinaesthetic and oral sensorimotor stimulation is associated with earlier achievement of independent oral feeding compared to the use of OS alone. On account of ethical concerns, we did not use a control group without intervention, as it is known that OS shortens the time to transition from tube feeding to independent oral feeding,29,30 and therefore we considered it would be inacceptable to deprive a group of neonates from this intervention.
We conducted the study in a sample of very preterm infants (27-32 weeks of gestation), who tend to have very prolonged lengths of stay, among other factors, due to their difficulty in achieving adequate oral feeding,1 which is why it is important to identify effective interventions to improve oral feeding in this gestational age group.
Effect of the combined intervention on the time to achievement of independent oral feedingMost studies to date have assessed the effect of OS in PTNBs compared to a control group that did not undergo intervention.18 Few studies compare the effect of combined stimulation versus oral stimulation alone in the achievement of independent oral feeding, as we have done. Three studies conducted by the same research group31–33 compared 4 groups of PTNBs that received tactile/kinaesthetic stimulation, OS, combined T + K + OS, and no intervention (control group). The authors found that combined stimulation interventions (T + K + OS) had a greater impact on the transition to oral feeding, oral skills31,33 and the coordination of sucking and swallowing and of breathing and swallowing,32 although all of them, when used independently, improved feeding skills compared to the control group.
Other authors that used combined interventions different from the one used in our study also found that combined stimulation interventions were more effective compared to the absence of intervention. Rustam et al34 compared a control group of 55 PTNBs with an intervention group of 53 PTNBs, all delivered before 33 weeks of gestation, that underwent different sensorimotor stimulation interventions: physical therapy techniques to promote proper body flexion and alignment, orofacial stimulation and manoeuvres to improves suck-swallow-breathe synchrony, occupational therapy and psychomotor interventions to promote a developmentally supportive environment and engagement of the parents in the interventions to promote parent-infant bonding. The authors found that infants that participated in more than 1 session of any of these approaches achieved oral feeding in a significantly shorter time compared to infants in the control group. In a study conducted on 64 PTNBs delivered after 32 weeks of gestation, Nadar et al35 found that the group that underwent a physical therapy intervention (with massage, kinaesthetic exercises and OS for 5 days) had greater weight gains and achieved better coordination of sucking and swallowing compared to a group with an equal number of infants that underwent multisensory stimulation (auditory, tactile, visual and vestibular).
A recent systematic review36 including 19 studies (15 randomised, 1 quasi-randomised and 3 crossover randomised controlled trials) grouped interventions by type into 6 categories: non-nutritive sucking, non-nutritive sucking with auditory reinforcement, sensorimotor stimulation, oral support (support of cheeks and chin during feeding sessions), combined training (sensorimotor + non-nutritive sucking or OS) and nutritive sucking aimed at facilitating swallow mechanisms (placing a milk bolus on the tongue for 15 minutes or use of a controlled-flow vacuum-free bottle system to maintain continuous availability of milk at the level of the mouth for 20 minutes). The authors concluded that all these varied interventions, based on different principles and methods, were effective in improving sucking, although interventions based solely on non-nutritive sucking achieved poorer outcomes. Non-nutritive sucking with a pacifier offers benefits to PTNBs, such as stress and pain reduction, especially when combined with other measures of analgesia, such as sucrose administration,27 but is not as effective in improving sucking and may interfere with breastfeeding, which requires a different sucking mechanism.27 Thus, it is recommended that a finger be used for non-nutritive stimulation rather than a pacifier, as was done in our study, as there is evidence that this improves the sealing of the lips and the peristaltic action of the tongue.37
A possible explanation of the greater efficacy of combined stimulation (T + K + OS) observed in our study and previous works31–33 is that sensorimotor stimulation may improve alertness and promote the maturation of the neural pathways that regulate postural control of the head and neck and the coordination of breathing and swallowing, which in turn would have a positive impact on the feeding skills of the infant.
Effect of the intervention on the length of stayMost previous studies assessed the impact of OS on the length of stay compared to a control group that received no intervention. Both studies carried out on a small samples30,38 and those including larger numbers of PTNBs10,39 have found a significantly shorter length of stay in the OS group. On the other hand, Bache et al39,40 found a positive impact of OS on breastfeeding but not a significant reduction in length of stay.
A Cochrane review by Greene et al17 that included 19 randomised and quasi-randomised controlled trials comparing the effect of OS with the effect of non-oral stimulation or no intervention found that OS was associated with a reduction in the length of stay and the time of transition to oral feeding compared to standard care and non-oral stimulation, although the authors emphasised the poor methodological quality of many of the studies included in the review and urged for caution in the interpretation of its results.
The tactile and kinaesthetic protocol implemented in our study was a modification of the protocol used by Field et al in 1986.41 These authors compared 20 PTNBs that underwent three 15-minute sessions of stimulation a day for a period of 10 days with a control group of 20 PTNBs that received no intervention, and found that stimulation achieved a reduction in length of stay of 6 days. As we noted above, due to ethical considerations, our study did not include a control group with no intervention. When we compared combined stimulation (T + K + OS) with OS alone, we found the same reduction in length of stay as Field et al (6 days), although the difference between groups was not statistically significant.
Previous studies that, like ours, compared the effect of combined stimulation to the effect of OS in the length of stay31–33 did not find a significant reduction in the length of stay.
One of the limitations of the study is that the sample size was determined by the enrolment of participants through non-probability convenience sampling, which limits the generalization of the results. Another potential limitation is that due to its characteristics (intervention: combined vs oral stimulation), double-blinding was not possible. The only researcher that was not aware of the intervention received by each of the groups was the person that carried out the analysis of the data. Lastly, we were unable to assess the individual contribution of kinaesthetic and tactile stimulation to the overall effect of the intervention, as they were used in combination. At any rate, our findings may be relevant to clinical practice for optimising the care of these patients by shortening the time of transition from tube feeding to oral feeding.
ConclusionOur findings suggest that interventions combining tactile, kinaesthetic and oral sensorimotor stimulation may have a greater impact compared to OS alone, achieving a significant reduction in the days to independent oral feeding and therefore improving the quality of life of PTNBs. This approach could also reduce the length of stay, although the differences we found in this parameter were not statistically significant.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Hernández Gutiérrez MF, Díaz-Gómez NM, Jiménez Sosa A, Díaz Gómez JM, Domenech Martinez E. Eficacia de 2 intervenciones para la alimentación oral independiente en pretérminos. An Pediatr. 2022;96:97–105.
Previous presentation: partial results of this study were presented at the VI International Congress of Paediatric Nursing, May 16-18, 2018, Valencia, Spain.