Numerous studies have evinced the efficacy of exclusive enteral nutrition (EEN) to induce remission in paediatric-onset Crohn’s disease (POCD).1–4 The guidelines of the European Crohn's and Colitis Organization and the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ECCO-ESPGHAN) recommend the use of EEN combined with early initiation of immunosuppression in patients with mild to moderate forms of disease.5 However, there are no data on the long-term efficacy of this strategy in preventing or postponing the use of biological therapy.
Thus, to determine the proportion of our patients with POCD that require initiation of anti-tumour necrosis factor (TNF) therapy after achieving clinical remission with the aforementioned approach, we conducted the observational retrospective study presented in this article.
We reviewed the medical records of patients with POCD that were diagnosed in our unit between 2003 and 2017 and achieved clinical remission at onset with a combination of EEN and thiopurine drug therapy (azathioprine, mercaptopurine). We collected demographic, clinical and outcome data for these patients until February 2019 or their transition to the adult care unit.
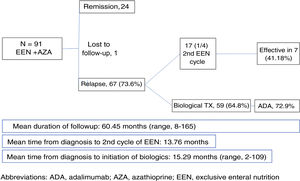
We included 91 patients (68.1% male; mean age at onset, 12.29 years, median age, 13 years; age range, 8 months–17 years) (Fig. 1). The mean duration of follow-up of patients in our unit was 60.45 months (range, 8–165 months). During this time, 66 of the 91 patients (72.53%) relapsed. The strategy used to manage the relapse in 17 patients (25.76%) was a second cycle of EEN, which was effective in 7 of them (41.18%). The mean time elapsed from diagnosis to the second cycle of EEN was 13.76 months (maximum, 110 months). During the follow-up, 65.6% of the patients required escalation of treatment to anti-TNF therapy due to failure of maintenance with thiopurines, with a mean time elapsed from onset to initiation of anti-TNF therapy of 15.29 months (median, 9 months). Of all patients that required biological therapy, 72.9% started with adalimumab (ADA). After a period of combined treatment (anti-TNF and thiopurines), the immunosuppressive treatment was discontinued in 42.2% of patients once they exhibited sustained clinical and endoscopic remission, thus switching to anti-TNF as monotherapy.
Despite the limitations intrinsic in the retrospective design of the study, the results obtained in a large sample of patients (n = 91 patients) show that although EEN is an effective approach for induction of remission in POCD, a successful-enough approach has yet to be established for subsequent maintenance to prevent the need of biological therapy in the medium term in a significant proportion of patients. Such an approach should involve more strict criteria for the definition of remission and a thorough evaluation of the latter so enable the establishment of more appropriate maintenance therapy.
FundingThis study did not receive any funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pascual Pérez AI, Pujol Muncunill G, Domínguez Sánchez P, Feo Ortega S, Martín de Carpi J. Duración de la remisión sostenida tras el tratamiento de inducción con nutrición enteral exclusiva y azatioprina en pacientes con enfermedad de Crohn. An Pediatr (Barc). 2021;94:252–253.
Previous presentation: This study was presented and received the award to the best brief oral communication in Gastroenterology/Nutrition at the XXVI Congress of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica; May 16–18, 2019; Santander, Spain.