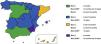
The actual meningococcal vaccine coverage in Spain exceeds official estimates, so the epidemiological trends that we measure do not correspond to the official immunization schedule covered by the state, but to the coverage achieved by parents and paediatricians that adhere to the schedule recommended by the Asociación Española de Pediatria (Spanish Association of Pediatrics).1 It is difficult to understand the decision of the Interterritorial Council to once again neglect the opportunity to update its meningococcal vaccination strategy for year 2022. There are also disparities between autonomous communities in Spain which, on one hand, accept the “unified” nationwide schedule but then complement it as they consider appropriate, giving rise to 4 different meningococcal vaccination schedules in Spain (Fig. 1). This state of affairs exacerbates existing inequities based on the residential zip code in the protection of children against meningococcal disease.2
Current schedules for vaccination against meningococcal disease in Spain. As of April 2022, there are at least 4 different meningococcal vaccination schedules, 3 of which offer greater coverage and diverge from the unified scheduled established by the Interterritorial Council of the National Health System, which constitutes a source of inequity based on the residential zip code.
Establishing the optimal schedule for vaccination against meningococcal disease is not an easy task, and there is wide variability within Europe alone, with differences that cannot be explained based on epidemiological or other logical criteria.3 In my opinion, given the uncertainty, the best approach is to offer the broadest possible coverage, including both serogroups B and ACWY and with vaccination of both infants and adolescents (Fig. 2). The decision in Spain of vaccinating adolescents against the ACWY serogroups (MenACWY vaccine) may be useful in the medium term, given based on the class effects of conjugate vaccines on carriers and the positive outcomes of this strategy observed in the United Kingdom. However, the current coverage rates cannot achieve indirect protection of unvaccinated individuals, except against group C. Once the herd immunity threshold can be reached through a high and sustained vaccination coverage in the cohort of adolescents vaccinated with MenACWY vaccine, it would be possible to do away with the doses given in infancy. In the case of group B, since vaccination has no impact on carriers, the vaccine will only offer direct protection. There are no data confirming the need of a booster dose of meningococcal B vaccine in older children or adolescents correctly vaccinated with the primary series in infancy, nor that a single dose at this time would be sufficient to maintain protection or to obtain the potential benefits of cross-protection described in the literature for this age group. However, vaccination of adolescents against group B meningococcus is important for their protection, as they are the age group with the second highest incidence of meningococcal disease and it is possible that these conjugate vaccines may offer a clinically significant degree of cross-protection against gonorrhoea, a disease that is exhibiting a marked surge for which there are no specific vaccines.
The most comprehensive schedule for child vaccination against meningococcal disease based on the vaccines currently available. The implementation of this schedule is subject to the indications detailed in the summary of product characteristics of each vaccine based on the age of administration and current epidemiological trends. Consult the corresponding text for a detailed explanation.
* According to current summaries of product characteristics, vaccination of infants aged less than 6 months requires at least a 2-dose primary series. Replacing the current dose of monovalent MenC vaccine at 4 months by a dose of MenACWY would suffice to achieve protection against group C. A clinical trial is currently underway to evaluate a 1 + 1 series of quadrivalent meningococcal tetanus toxoid conjugate vaccine (ACWY-TT) at ages 4 and 12 months. The progressive elimination of the infant MenACWY doses could be considered once the herd immunity threshold is achieved through a high and sustained MenACWY vaccine coverage in adolescents, as was done with the administration of MenC vaccine in infants in the past.
& The second dose of MenB could be administered as early as age 3 months in vaccination schedules with a 3-dose primary series. In 2-dose schedules, the doses must be given at least 8 weeks apart, according to the summary of product characteristics.
$ This dose could be given at 12 or 14 years at the same time as other vaccines. There is still no data supporting the need for a booster dose against serogroup B in children who received the full primary vaccination series, nor that a single dose at this time would be sufficient to maintain protection or to obtain the potential benefits of cross-protection described in the literature for this age group.
Meningococcal disease is a single illness, but it is caused by different serogroups, and today safe and effective vaccines are available that offer coverage against the groups involved in nearly all cases that occur in Spain. If we really want to control meningococcal disease and are serious about pursuing the goal of a meningitis-free world by 2030, a target set by the World Health Organization, the official immunization schedule of Spain should take a comprehensive approach, offering coverage against every serogroup for which it is available and protecting infants as well as adolescents.4 Each case of meningococcal disease in Spain should be considered a public health failure.
Conflict of interestsFM-T has received fees as an advisor, consultant or lecturer from Biofabri, GSK, Pfizer Inc., Sanofi Pasteur, MSD, Seqirus, Novavax and Janssen in areas unrelated to the subject of this article. FMT has been the principal investigator in clinical trials funded by the same pharmaceutical companies in addition to Ablynx, Regeneron, Roche, Abbott and MedImmune, for which the fees were paid to the institution FMT is affiliated to.