Pertussis is a respiratory infection caused by bacteria of the genus Bordetella, mainly pertussis and parapertussis species. Despite the high vaccination coverage in developed countries, it is considered a re-emerging disease that is also underreported and underdiagnosed, especially in patients who do not require hospital referral.
Material and methodsDescriptive, prospective and multicentre study of pertussis diagnosis and contact investigation in 17 primary care paediatric clinics through collection of samples for polymerase chain reaction (PCR) testing over a period of 4 years and after the implementation of routine vaccination against pertussis during pregnancy.
ResultsPertussis was diagnosed in a total of 50 patients; the estimated incidence in these years was higher compared to previous rates in the paediatric age group. Fourteen percent of the cases occurred in children aged less than 1 year. The mean age was 6.7 years. Cough was present in 100% of cases, followed in frequency by vomiting and rhinorrhoea. Only 1 patient required hospital admission, and none died or developed complications. B. pertussis was the predominant causative agent. Only 40% knew the source of infection. In 26% of the cases, pertussis was confirmed in contacts of the patient by PCR, and in 46% it was suspected based on the clinical presentation but without microbiological confirmation.
ConclusionsAccess to diagnostic tests (PCR) for pertussis in primary care allows us to optimise its diagnosis and treatment, to break the chain of transmission, to know the real incidence rates and to assess the impact of routine vaccination of pregnant women on this disease.
La tos ferina es una infección respiratoria causada por bacterias del género Bordetella, principalmente por las especies pertussis y parapertussis. A pesar de las altas coberturas vacunales en países desarrollados, está considerada como una enfermedad reemergente existiendo, además, una infranotificación y un infradiagnóstico especialmente en los pacientes que no precisan derivación hospitalaria.
Material y métodosEstudio descriptivo, prospectivo y multicéntrico de diagnóstico de casos de tos ferina, así como el estudio de sus contactos en 17 consultas de pediatría de atención primaria mediante la toma de muestras para realización de técnicas de PCR (reacción en cadena de la polimerasa) a lo largo de cuatro años y tras la implantación de la vacunación sistemática de la tos ferina en el embarazo.
ResultadosSe diagnostican un total de 50 pacientes; la tasa de incidencia estimada en estos años fue superior a las publicadas en edad pediátrica. Un 14% de los casos sucedieron en menores de 1 año. La media de edad fue de 6,7 años. La tos estuvo presente en el 100% de los casos, seguida de los vómitos y rinorrea como síntomas más frecuentes. Sólo 1 paciente precisó ingreso y ninguno falleció ni presentó complicaciones. B. pertussis fue el agente causal predominante. Sólo un 40% conocía la fuente de contagio. En un 26% de los casos se comprobó mediante PCR tos ferina en sus contactos y en un 46% se sospechó clínicamente, aunque sin confirmación microbiológica.
ConclusionesEl acceso a pruebas diagnósticas (PCR) para tos ferina en Atención Primaria permite optimizar su diagnóstico y tratamiento, cortar la cadena de transmisión, conocer las tasas de incidencia reales y valorar el impacto de la vacunación sistemática de las embarazadas en esta enfermedad.
Pertussis is an acute respiratory infectious disease caused by Bordetella bacteria. The most frequent causative agent is Bordetella pertussis.
In addition to B. pertussis, another 3 Bordetella species are known to cause disease in humans: B. parapertussis, B. holmesii and B. bronchiseptica. The natural history of infection by B. parapertussis is similar to that of pertussis, but milder1–3. It also exhibits a cyclical trend with outbreaks every 3–5 years.
The disease has an insidious onset that cannot be distinguished from other mild respiratory diseases, and this is the initial or catarrhal stage, when the disease is most contagious. However, the diagnosis does not usually occur until 2–3 weeks later, when the cough progresses to the paroxysmal stage and can be accompanied by a distinctive “whooping” in inspiration. Episodes with coughing fits increase both in frequency and severity for 4–8 weeks and then gradually improve during the convalescence stage, which may last another 2–4 weeks4–6.
The clinical presentation of pertussis varies with age and vaccination status. In adolescents and adults, the disease may be mild and not be identified as pertussis. Infants aged less than 6 months, unvaccinated or with incomplete vaccination, are at greater risk of complications and death7.
Different seroepidemiological studies8,9 have evinced widespread circulation of B. pertussis worldwide independently of vaccination schedules and coverage. In Spain, where the vaccination coverage exceeds 95%, the incidence of pertussis increased from 2010 to 2015, exhibiting a bimodal distribution with peaks in infants aged less than 6 months that had not started or completed primary vaccination, and in adolescents in adults due to the immunity acquired through vaccination or natural infection waning over time8,9.
Possible explanations for this increase in incidence include the increased access to rapid diagnostic tests, which has improved reporting of the disease, the waning of the protective effect of vaccination, increased circulation of B. pertussis strains, genetic changes in circulating strains and a potentially lower effectiveness of the acellular vaccine compared to the whole-cell vaccine10–13.
However, there is evidence suggesting that the incidence of pertussis is much greater than it would seem based on the data obtained through passive surveillance systems14,15, due to underreporting and underdiagnosis, especially in patients who do not require referral to the hospital, where the diagnosis would be confirmed, due to their age or the mildness of their symptoms.
Most susceptible patients are fully or partially vaccinated, and they are not likely to develop severe pertussis. In most cases, they are managed by their primary care (PC) paediatricians, especially in the early stages of disease, which are, as we mentioned, the most contagious.
Thus, it would be beneficial for the means required for diagnosis of pertussis to be available in the PC setting. At present, the gold standard of diagnosis is a polymerase chain reaction (PCR) test for detection of B. pertussis/parapertussis in a nasopharyngeal aspirate or throat swab sample. The PCR test allows rapid diagnosis and is also highly sensitive (90,7–97%) and specific (93%–100%) compared to culture (sensitivity, 58%–64%; specificity, 100%)16. However, there is substantial variation between autonomous communities in Spain in the use of PCR for diagnosis of pertussis, both in terms of availability and the need to refer patients to the hospital for its performance, which delays and hinders its diagnosis in PC17.
The aim of our study was to demonstrate that access to PCR testing and the capacity to obtain samples for the purpose in paediatric PC clinics allows identification of pertussis cases, thus contributing to improved reporting and a more accurate estimation of the actual incidence, optimization of antibiotic use and initiation of treatment in the early stages of disease, thus halting the chain of transmission earlier.
Sample and methodsWe conducted a multicentre prospective descriptive study of paediatric patients aged 0–14 years that received a diagnosis of pertussis based on the use of specific PCR tests for detection of B. pertussis/parapertussis in 17 PC clinics in a 4-year period (June 1.2016 to May 31, 2020).
The study was conducted in 6 autonomous communities (ACs) in Spain with the collaboration of PC paediatricians that volunteered to participate. In each AC, we counted with the collaboration of the regional microbiology reference laboratory.
We provided the parents or legal guardians of all patients and informational leaflet inviting them to participate in the study, and a consent form that was signed prior to inclusion in the study.
The study was approved by the competent ethic committees of each participating AC before its initiation.
Table 1 lists the ACs represented in the study, the number of paediatricians that participated in each of them, and the total number of children resulting from adding up the caseloads of all the paediatricians that collaborated in each AC, represented as “n” (total number = 16 906). We only included the caseloads and cases managed by PC paediatricians that remained in the study through the 4-year follow-up. Routine vaccination against pertussis in pregnant women had been introduced in every AC by the end of 2015.
The diagnosis of pertussis was based on the pertussis surveillance protocol of the National Network of Epidemiological Surveillance of Spain (known as RENAVE) and considering suspected cases (patient meeting the clinical case definition), probable cases (patients meeting the clinical criteria and known contact with a laboratory-confirmed pertussis case 6 to 20 days before the onset of symptoms) and confirmed cases (patients meeting both clinical and laboratory criteria)18.
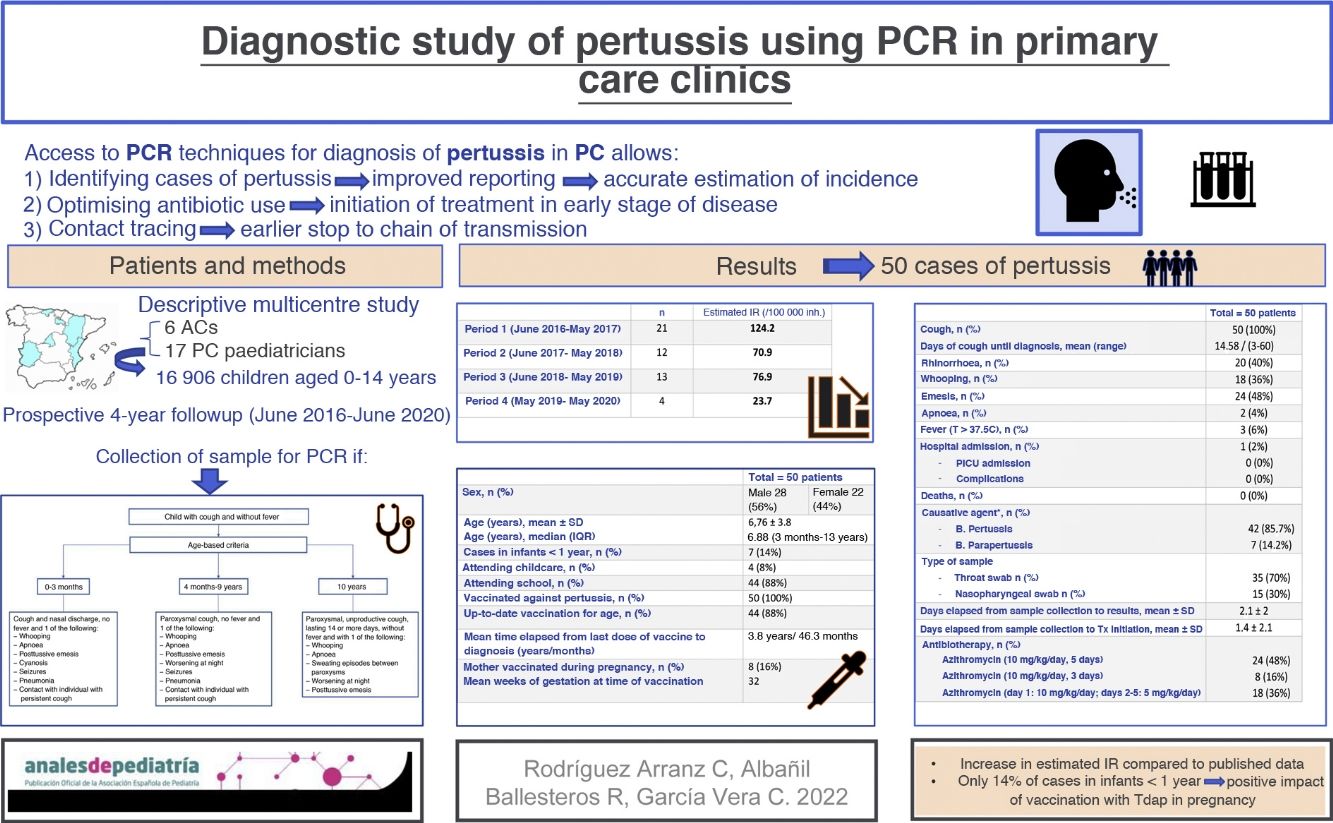
The clinical criteria applied to define a suspected case were the updated Global Pertussis Initiative criteria published in 201219, which are summarised in Fig. 1.
Clinical criteria for suspected pertussis19.
Adapted from: Cherry JD et al. Clin Infect Dis. 2012:54;1756–1764.
Every patient that met the clinical and/or epidemiological criteria underwent microbiological testing for confirmation of the infection by detection of Bordetella pertussis/parapertussis nucleic acid in a clinical sample (throat swab/aspirate) by PCR. Samples were collected by qualified professionals (paediatrician or nurse) in the PC clinic.
The study included every child aged 0–14 years in the caseloads of collaborating paediatricians in whom, after pertussis was suspected based on the clinical criteria noted above, the diagnosis was confirmed by PCR. We decided to include a single probable case in which the diagnosis was strongly suspected in a patient with close contact with a case confirmed by PCR, in whom the negative result of PCR was interpreted as a false negative because the test was conducted outside the time window in which it is sensitive20.
We excluded patients if any of the following applied at the time of sample collection: 1) poorly controlled bronchial asthma with persistent cough, 2) abnormal sounds on auscultation 3) presence of another diagnosed respiratory infection (sinusitis, pneumonia, bronchitis/bronchiolitis).
All patients with a pertussis diagnosis, in addition to their close and high-risk contacts, received antibiotherapy and the applicable respiratory isolation precautions implemented, advising adherence to current guidelines18,21. All the cases were notified to the epidemiological surveillance system.
ResultsA total of 50 cases of pertussis were detected during the 4-year follow-up. The study was divided in 4 periods, as can be seen in Table 2. The table includes the estimated incidence rate (IR) of pertussis per 100 000 inabitants aged 0–14 years for each period.
Cases of pertussis and estimated incidence rate per period.
| n | IR (per 100 000 inh.) | |
|---|---|---|
| Period 1(June 1, 2016–May 31, 2017) | 21 | 124.2 |
| Period 2(June 1, 2017–May 31, 2018) | 12 | 70.9 |
| Period 3(June 1, 2018–May 31, 2019) | 13 | 76.9 |
| Period 4(May 1, 2019–May 31, 2020) | 4 | 23.7 |
inh, inhabitants; IR, incidence rate in children aged 0–14 years.
No cases were detected in the last 4 months of the follow-up, which overlapped with the lockdown implemented on account of the SARS-CoV-2 pandemic.
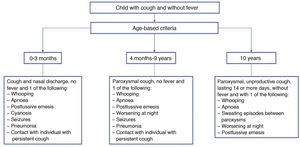
To compare our findings with the published incidence data, Fig. 2 presents the temporal trends by age group in Spain based on the data published by RENAVE13,22, which we will discuss in the discussion section.
Incidence by age group (1998–2016)22.
Table 3 presents the general characteristics of the cases of pertussis.
General characteristics of the cases.
| Total = 50 patients | |
|---|---|
| Sex, n (%) | Male 28 (56%) |
| Female 22 (44%) | |
| Age (years), mean ± SD | 6.76 ± 3.8 |
| Age (years), median (IQR) | 6.88 (3 months–13 years) |
| Cases in infants <1 year, n (%) | 7 (14%) |
| Attending childcare, n (%) | 4 (8%) |
| Attending school, n (%) | 44 (88%) |
| Vaccinated against pertussis, n (%) | 50 (100%) |
| Received doses of vaccine, n (%) | 5 doses: 15 (30%) |
| 4 doses: 26 (52%) | |
| 3 doses: 2 (4%) | |
| 2 doses: 6 (12%) | |
| 1 dose: 1 (2%) | |
| Up-to-date vaccination for age, n (%) | 44 (88%) |
| Mean time elapsed from last dose of vaccine to diagnosis (years/months) | 3.8 years/46.3 months |
| Mother vaccinated during pregnancy, n (%) | 8 (16%) |
| Mean weeks of gestation at time of vaccination | 32 |
| Mean GA (weeks) at birth | 39 |
GA, gestational age, IQR, interquartile range, SD, standard deviation.
There was a predominance of male versus female patients (56% vs 44%); the mean age at diagnosis was 6.7 ± 3.8 years, and the median was 6.88 years, with a range of 3 months to 13 years.
Seven cases (14%) were diagnosed in infants aged less than 1 year (1 aged less than 3 months, 1 aged 6 months, 2 aged 8 months, 2 aged 9 months and 1 aged 11 months).
Of all cases, 88% occurred in children that were enrolled in school or a childcare centre.
In 100% of the cases, the patient had received at least 1 dose of the vaccine against pertussis, although 12% had incomplete vaccination for their age based on the current immunization schedule. The mean time elapsed between the administration of the most recent dose and diagnosis was 3.8 ± 2.5 years.
In most cases (84%), the mother had not been vaccinated during pregnancy, as the children were older and had been born before routine vaccination of pregnant women was recommended or implemented in Spain.
The 8 cases in which the mother had been vaccinated (16%) corresponded to the 7 patients aged less than 1 year and 1 patient aged 1.5 years. Therefore, in every case detected in children under 1 year, the mother had been vaccinated during pregnancy.
Table 4 presents the data on the clinical manifestations and microbiological test results in patients given a diagnosis of pertussis during the follow-up.
Clinical characteristics and microbiological test results in cases of pertussis.
| Total = 50 patients | |
|---|---|
| Cough, n (%) | 50 (100%) |
| Days of cough until diagnosis, mean (range) | 14.58 (3–60) |
| Rhinorrhoea, n (%) | 20 (40%) |
| Whooping, n (%) | 18 (36%) |
| Emesis, n (%) | 24 (48%) |
| Apnoea, n (%) | 2 (4%) |
| Fever (T > 37.5 °C), n (%) | 3 (6%) |
| Hospital admission, n (%) | 1 (2%) |
| PICU admission | 0 (0%) |
| Complications | 0 (0%) |
| Deaths, n (%) | 0 (0%) |
| Causative agenta, n (%) | |
| B. Pertussis | 42 (85.7%) |
| B. Parapertussis | 7 (14.2%) |
| Type of sample | |
| Nasopharyngeal swab, n (%) | 35 (70%) |
| Nasopharyngeal aspirate, n (%) | 15 (30%) |
| Days elapsed from sample collection to results, mean ± SD | 2.1 ± 2 |
| Days elapsed from sample collection to Tx initiation, mean ± SD | 1.4 ± 2.1 |
| Antibiotherapy, n (%) | |
| Azithromycin (10 mg/kg/day, 5 days) | 24 (48%) |
| Azithromycin (10 mg/kg/day, 3 days) | 8 (16%) |
| Azithromycin (day 1: 10 mg/kg/day; days 2-5: 5 mg/kg/day) | 18 (36%) |
SD, standard deviation; T, body temperature; Tx, treatment.
Cough was present in 100% of cases, with a mean of 14 days elapsed from onset to diagnosis (maximum, 60 days; minimum, 3 days). The second most frequent symptom was vomiting (48%), followed by rhinorrhoea (40%) and stridor/whooping (36%). Increased body temperature and apnoea were only detected in 6% and 2% of cases, respectively.
Only 1 patient, an infant aged 8 months, required hospital admission, with a length of stay of 5 days. None of the patients required admission to the paediatric intensive care unit.
All patients had favourable outcomes without complications.
The most frequently identified causative agent was B. pertussis, found in 85.7% of the cases. The sample used for testing was a nasopharyngeal swab in 70% of these cases and nasopharyngeal aspirate in 30%.
The turnaround time was short, with test results available a mean of 2 days after sample collection, which allowed early initiation of antibiotherapy.
The selected antibiotic was azithromycin in 100% of cases, usually with prescription of a 5-day course.
Table 5 presents the clinical and microbiological data corresponding to patients aged less than 1 year.
Clinical characteristics and microbiological test results in cases in patients aged less than 1 year.
| Total = 7 patients | |
|---|---|
| Cough, n (%) | 7 (100%) |
| Days of cough until diagnosis, mean (range) | 11 ± 6 |
| Rhinorrhoea, n (%) | 5 (71.4%) |
| Whooping, n (%) | 2 (28.5%) |
| Emesis, n (%) | 2 (28.5%) |
| Apnoea, n (%) | 1 (14.2%) |
| Fever (T > 37.5 °C), n (%) | 0 (0%) |
| Hospital admission, n (%) | 1 (14.2%) |
| PICU admission | 0 (0%) |
| Complications | 0 (0%) |
| Deaths, n (%) | 0 (0%) |
| Causative agent, n (%) | |
| B. Pertussis | 5 (71.4%) |
| B. Parapertussis | 2 (28.5%) |
| Type of sample | |
| Nasopharyngeal swab, n (%) | 3 (42.8%) |
| Nasopharyngeal aspirate n (%) | 4 (57.1%) |
| Mother vaccinated during pregnancy, n (%) | 7 (100%) |
PICU, paediatric intensive care unit; T, body temperature.
A contact investigation was conducted in every case, the results of which are presented in Table 6.
Contact investigation.
| n (%) | Total = 50 patients | |
|---|---|---|
| YES, 20 (40%) | NO, 30 (60%) | |
| Index case identified corresponded to: | ||
| Household member | 14 (70%) | |
| Other relatives | 2 (10%) | |
| Other (school, friends) | 4 (20%) | |
| Number of cases with MC contacts, which were | 13 (26%) | |
| Household member | 11 (87%) | |
| Other relatives | 0 (0%) | |
| Other (school, friends) | 2 (13%) | |
| Number of cases with contacts not MC, which were | 20 (40%)a | |
| Household member | 17 (85%) | |
| Other relatives | 6 (30%) | |
| Other (school, friends) | 0 (0%) | |
MC, microbiologically confirmed.
The index case was identified 40% of the cases, and in 70% of these cases it was a member of the household (parents, siblings), in 20% a classmate and in 10% other relatives (grandparents, cousins. In the other 60% of cases, it could not be determined how patients had acquired the infection.
In 26% of the patients, contact tracing identified contacts with symptoms compatible with pertussis in whom the diagnosis could be confirmed by PCR. Eighty-seven percent of these contacts were parents or siblings of the patient, and 13% were classmates.
In 40% of the patients, contact tracing identified contacts in whom pertussis was suspected based on the clinical presentation but in whom the diagnosis could not be confirmed, for reasons that were not specified. Most of these contacts with suspected pertussis (85%) were also household members.
DiscussionPertussis is a bacterial respiratory infection that continues to be a public health problem. Despite the high vaccination coverage rates in developed countries, it can be considered a re-emerging disease23.
In Spain, the data published by RENAVE obtained through current notification systems evinced a high incidence in infants (age <1 year and especially age <3 months) that had not completed primary vaccination or had not yet started it due to their young age. These patients are usually referred to hospital due to the severity of their symptoms, where they undergo microbiological testing, chiefly with PCR, to confirm the diagnosis.
The most recent surveillance report published by the RENAVE, Instituto de Salud Carlos III and the Ministry of Science and Innovation corresponds to the 2017–2018 period. The latest epidemic cycle started in 2014, peaking in 2015 with an incidence of 18.04 cases per 100 000 inabitants, and started to decline in 2016 (IR = 11.60), with the decreasing trend continuing in 2017 (IR = 10.6) and 2018 (IR = 7.76)22.
The IRs estimated with data from passive surveillance systems for 2016 (first year under study) were, with the exception of the group aged less than 1 year, clearly lower compared to the IRs estimated in our study with an active case search strategy: 49.3 (1–4 years), 53.9 (5–9 years) and 40.5 (10–14 years)13 compared to 124.2 in our study.
This was again the case in 2017 and 2018, also with the exception of infants aged less than 1 year22: 56.7 and 39.5, respectively (1–4 years), 62.3 and 30.3 (5–9 years) and 37.1 and 33.0 (10–14 years) compared to 70.9 and 76.9 in our study.
Final data for years 2019 and 2020 have yet to be published by RENAVE.
It is important to take into account that the symptoms presented by patients with pertussis are often nonspecific and may not be distinguished from other respiratory illnesses, especially in children older than 1 year, who in most cases have received more than 1 dose of the vaccine. A retrospective hospital-based study in Spain found that 33.7% of patients with pertussis diagnosed in the emergency department had visited the department twice or more and had initially received other diagnoses, such as upper respiratory tract infection, cough, bronchiolitis/bronchitis or laryngitis, among others24,25.
In the PC setting, in addition to the initial lack of suspicion, there is substantial variation between ACs in the access to diagnostic methods, such as PCR17, which contributes to patients with pertussis receiving other diagnoses or empiric antibiotherapy without microbiological confirmation, contributing to underreporting and the underestimation of the incidence of pertussis in children who do not need referral or admission to hospital, which is the case of most patients aged more than 1 year.
Recent hospital-based studies16,24 demonstrate the usefulness of PCR for diagnosis of pertussis, but to date there are no published data on its use in PC paediatrics practice. Our study demonstrates that access to diagnostic methods like PCR in paediatric PC settings can contribute to knowing the actual incidence of this disease, as we found incidence rates that were higher than previously reported, despite the follow-up taking place in the years when the most recent epidemic cycle, which peaked in 2015, was waning.
However, unexpectedly, our study only identified 7 cases in infants aged less than 1 year during the 4-year follow-up, which shows that the contribution of this age group to estimated incidence rates is low, and we only found a history of maternal vaccination during pregnancy in 8 of the 50 detected cases, which could be explained by the positive impact of vaccination against pertussis during pregnancy.
In Spain, the Interterritorial Council of the National Health System endorsed the recommendation of vaccination against pertussis during pregnancy in June 2015, and this guideline was extended to all ACs in 2016. At the outset of our study, all participating ACs routinely vaccinated pregnant women against pertussis. The estimated nationwide vaccination coverage in pregnant women was 81.6% in 2016, increasing in 2018 and 2019 to 82.9% and 83.6%, respectively25.
The preliminary analysis of the impact of vaccination of pregnant women published in 2018 by the Spanish Ministry of Health suggests an impact in the form of a reduction in the incidence of disease in infants aged less than 3 months, with earlier evidence of this decrease in ACs with earlier implementation of vaccination during pregnancy13.
Recent studies corroborate this positive impact of vaccination during pregnancy on paediatric hospital admissions26 and a decreased risk of pertussis in the early months of life27.
As evinced by contact tracing, adolescents and adults are a reservoir from which the disease can be transmitted to infants and newborns. For this reason, the routine immunization schedule proposed by the Asociación Española de Pediatría (Spanish Association of Pediatrics, AEP) recommends administration of a dose of TdaP in adolescence, a strategy that has been already implemented in other European countries28.
As regards treatment, there is an unwarranted tendency to treat children aged more than 6 months with a 5-day course of azithromycin at a dose of 10 mg/kg/day, which would only be indicated in younger infants. Current management protocols call for a dose of 10 mg/kg/day for 3 days or longer 5-days courses in which a lower dose of 5 mg/kg/day is given between days 2 and 5 of treatment18,21.
To conclude, we ought to highlight the importance of making diagnostic tests for confirmation of pertussis, such as PCR, accessible at the PC level, as, in addition to optimising diagnosis, it allows appropriate isolation of cases while contagious, early initiation of antibiotherapy and identification of close contacts that could benefit from treatment, thus stopping the chain of transmission. Furthermore, it would make it possible to calculate the actual incidence of pertussis.
FundingThis study received the 2016 Asociación Española de Pediatría-Fundación Pediatría y Salud (AEPap–FPS) grant for research in primary care paediatrics.
Conflicts of interestThe authors have no conflicts of interest to declare.
We want to express our heartfelt appreciation to all the paediatricians that collaborated with the study: Beatriz Rituerto Gómez, Ana Castroviejo Gandarias, José Vicente Bernad Usoz, Jaime Tella Madorrán, Irene Calavia Redondo, María Elena León Angós, Teresa Arana Navarro, Teresa Cenarro Guerrero, María Eulalia Muñoz Hiraldo, Beatriz Acosta Navas, María José Martínez Chamorro, Marta Dapena Archilés, Carlos Labordena, Eva Bono, Ana Montañés, Ana Cubero Santos and Ana Grande Tejada. We also thank the specialists and technicians involved in the study at the microbiology reference laboratory of each autonomous community.