The aim of the study was to evaluate the diagnostic performance of the item concerning physical activity of the Global Initiative for Asthma (GINA) asthma control questionnaire for detection of exercise-induced bronchoconstriction (EIB) in children and adolescents.
Material and methodsWe divided participants (aged 6–18 years) with a diagnosis of asthma into two groups according to the GINA severity classification: mild/moderate asthma (MMA) and severe therapy-resistant asthma (STRA). We collected anthropometric, clinical and functional data (spirometry) and performed an EIB test. We used item 4 of the GINA questionnaire regarding exercise-induced symptoms to assess the diagnostic power of this instrument.
ResultsWe included 40 patients (17 with MMA and 23 with STRA) with a mean age of 11.3 years and a mean FEV1z-score of –0.33, of who 13 (32.5%) were classified as having uncontrolled asthma. Of the patients with uncontrolled asthma, 7 (53.8%) exhibited a decrease in the FEV1 after the EIB test. We found a higher frequency of EIB in participants with FEV1 z-score values of less than –1.0 compared to those with a z-score of –1.0 or greater (P = .05). There were no significant differences in the frequency of EIB based on disease severity and control. We also found no association of item 4 (GINA) with EIB. The area under the ROC curve demonstrated that the discriminative power of the GINA questionnaire for the detection of EIB is inadequate (P = .41), with sensitivity of 42.1% and specificity of 57.1%.
ConclusionsThe item concerning physical activity in the GINA questionnaire has insufficient diagnostic power to detect EIB in children and adolescents with asthma.
El objetivo del estudio fue evaluar el rendimiento diagnóstico de la pregunta relacionada con la actividad física del cuestionario de control del asma de la Global Initiative for Asthma (GINA) para la detección de la broncoconstricción inducida por el ejercicio (BIE) en niños y adolescentes.
Materiales y métodosSe dividió a pacientes (de 6 a 18 años de edad) con diagnóstico de asma en dos grupos de acuerdo a la clasificación de gravedad según el cuestionario GINA: asma leve/moderado (ALM) y asma grave refractaria (AGR). Se recogieron datos antropométricos, clínicos y funcionales (espirometría) y se realizó prueba de broncoprovocación con esfuerzo. Se utilizó la cuarta pregunta del cuestionario GINA, concerniente a los síntomas asociados al esfuerzo físico, para evaluar el rendimiento diagnóstico de este instrumento.
ResultadosSe incluyó a 40 pacientes (17 con ALM y 23 con AGR) con una edad media de 11,3 años y un z-score medio de FEV1 de –0,33, de los que 13 (32,5%) se clasificaron como casos de asma no controlada. De los pacientes con enfermedad no controlada, 7 (53,8%) mostraron caídas en el FEV1 tras la prueba de esfuerzo. Se observó una mayor frecuencia de BIE en sujetos con z-score de FEV1 < –1,0 en comparación con aquellos con un z-score ≥ –1,0 (p = 0,05). No hubo diferencias significativas en la frecuencia de BIE en base a la gravedad o el control de la enfermedad. Tampoco se observó asociación entre la pregunta 4 (GINA) y la BIE. El área bajo la curva ROC evidenció que el poder discriminatorio del cuestionario GINA resulta insuficiente para detectar la BIE (p = 0,41), con una sensibilidad del 42,1% y una especificidad del 57,1%.
ConclusionesLa pregunta concerniente al ejercicio físico en el cuestionario GINA carece del poder diagnóstico necesario para detectar la BIE en niños y adolescentes asmáticos.
Asthma is a chronic respiratory disease characterized by airway obstruction. Its prevalence in children is high, and it is a major cause of paediatric hospital admission worldwide. It manifests with wheezing, dyspnoea and dry cough, especially at night and/or early morning.1
Multiple mechanisms may trigger asthma exacerbations, including physical exertion, anxiety and environmental factors. Exercise-induced bronchoconstriction (EIB) is defined as transient airway obstruction during or following physical activity, evinced by a decreased forced expiratory volume in 1 s (FEV1) combined with signs and symptoms of the disease. Approximately 40%–90% of individuals with asthma have EIB.2 Exercise challenge testing is considered the gold standard to detect EIB, as it assesses bronchial hyperresponsiveness. However, this method requires considerable cooperation from the patient and carries risks, including development of severe bronchospasm and falling on the treadmill, especially for young children.2
Thus, questionnaires may offer practical, simple and feasible means to identify the presence of EIB. However, currently available instruments3–8 seem to have a low-to-moderate sensitivity (13%-71%) and specificity (47%-70.7%) in asthmatic children and adolescents, leading to false positives or false negatives in the detection of these changes. Therefore, no available instrument to date has exhibited an adequate diagnostic yield for detection of EIB in the paediatric population.9 In a study conducted by Westergren et al.,10 the use of the question ‘present feeling that asthma restrains physical activity’ proved insufficient to detect EIB. Similarly, a recent study investigated the use of a questionnaire developed by the Global Initiative for Asthma (GINA) to predict diagnosis of asthma based on the results of the methacholine challenge.11 Once again, the results showed a weak-to-moderate diagnostic accuracy (70.2% sensitivity and 49.1% specificity) for the item regarding physical activity in the clinical scale. Notwithstanding, this instrument is used widely in clinical practice to monitor asthma control. To the best of our knowledge, to date, no study has investigated the use of the fourth item of the GINA questionnaire (limitations of activity due to asthma) for detection of EIB through comparison with the results of an exercise challenge test.
Thus, considering the limitation of physical activity brought on by the disease and the fact that the GINA questionnaire is a simple and widely used tool for monitoring the control of disease, we considered that evaluating the diagnostic yield of the item of the GINA questionnaire that assesses the presence of symptoms in the context of physical activity for detection of EIB in children and adolescents was justified. Thus, the aim of our study was to evaluate the diagnostic performance of the GINA questionnaire item on physical activity for detection of EIB in children and adolescents. To this end, we recruited patients with mild-to-moderate asthma (MMA) and severe therapy-resistant asthma (STRA). We also analysed the association in our sample between a positive result of the exercise challenge test and asthma severity, disease control and pulmonary function.
Patients and methodsWe performed a cross-sectional study including children and adolescents of both sexes aged 6–18 years with a diagnosis of asthma followed up by the paediatric pulmonology outpatient clinic of our hospital between March 2016 and September 2018. We excluded patients with osteoarticular and musculoskeletal abnormalities, unable to perform a treadmill exercise test or with manifestations suggestive of an airway infection.
The diagnosis of asthma and the classification of disease severity (mild/moderate to severe) were based on the GINA criteria.12 We divided patients into 2 groups: the STRA group and the MMA group. The criteria for membership in the STRA group were asthma requiring GINA steps 4–5 treatment (budesonide ≥ 800 mg/day or equivalent, associated with long-acting β2-agonist; continuous use of an oral corticosteroid; or omalizumab) or presence of uncontrolled disease or disease requiring that level of treatment be controlled. Uncontrolled disease was defined as: 1) persistent symptoms or Asthma Control Test (ACT) score greater than 20 (for more than 3 months); 2) acute exacerbations (with at least two intensive care unit [ICU] admissions or 2 courses of oral corticosteroids in the past 12 months); or 3) non-reversible airflow obstruction even after steroid therapy. The criteria for membership in the MMA group were: recurrent episodes of shortness of breath, cough, wheezing, tightness in the chest, or abnormal pulmonary function (reduced FEV1 and FEV1/forced vital capacity [FVC] ratio, or FEV1 increasing by at least 12% of the baseline value after administration of a bronchodilator), undergoing GINA steps 1–3 treatment, and a duration of follow-up at the clinic of at least 3 months. We also used the 4 clinical items of the GINA questionnaire to stratify patients based on the level of disease control. We divided patients into the following groups based on the asthma control score: controlled asthma (4 negative answers); partially controlled asthma (1–2 negative answers) and uncontrolled asthma (3–4 positive answers).
We calculated the sample size necessary to achieve an 80% sensitivity/specificity for the GINA questionnaire item with a precision of 0.10 assuming a prevalence of EIB of 70%.2 We used a nomogram to estimate the sensitivity and specificity of diagnostic tests.13 We calculated that we needed a sample size of 40 patients. The study was approved by the local research ethics committee (protocol number 47845415.4.0000.5336). We obtained signed informed consent from the parents and/or legal guardians and a signed assent form from the participants.
Once children enrolled in the study, their parents completed the GINA asthma control questionnaire. Each patient underwent anthropometric measurement (weight and height) and a spirometry test. The exercise challenge test was performed afterward.
The anthropometric assessment consisted on obtaining 2 measures each for height and weight. Weight was measured with the patient standing barefoot in as little clothing as possible with a digital scale (G-Tech, Glass 1 F W, Rio de Janeiro, Brazil) accurate to 100 g. Height was measured with the patient barefoot standing upright with a portable stadiometer (AlturaExata, TBW, São Paulo, Brazil) accurate to 1 mm. We used the weight and height measurements to calculate the body mass index (BMI = weight in kg/[height in m2]), and then calculated the BMI z-score for age.14
We performed spirometry in adherence with the acceptability and reproducibility criteria of the American Thoracic Society and European Respiratory Society (ATS/ERS).15 Each test was performed with a KOKO spirometer (San Diego, California, USA). The parameters measured by means of spirometry were the forced vital capacity (FVC), FEV1, FEV1/FVC ratio, and forced expiratory flow between 25% and 75% of FVC (FEF25−75) expressed in litres (absolute value) and z-score values calculated with an international reference equation.16
GINA questionnaires are used worldwide for evaluation of asthma control and severity. The assessment of asthma control includes four clinical items, one of which addresses asthma in relation to physical activity (the specific wording is “any activity limitation due to asthma?”).12 We asked parents or legal guardians this question in reference to the 4 weeks preceding the interview.
We conducted exercise challenge tests in a safe and calm environment, with a temperature of 20 °C–24 °C, dry air with 10 mgH2O/L and a relative air humidity of approximately 60%. All tests adhered to the American Thoracic Society (ATS) recommendations17 and were monitored by physical therapists and the physician in charge to ensure the safety of participants. All participants performed a spirometry test before the exercise challenge (without administration of a bronchodilator) and repeated the test immediately after the challenge and at 5, 10, 15, and 30 min. The exercise challenge was performed on a KT-10400 treadmill (Imbramed, São Paulo, Brazil) with an adjustable incline (15%) and speed. We increased the speed until the participant reached a heart rate of 80% of the established maximum (220 – age in years).17 From that moment, the participant was expected to continue exercising at that level for additional 4−6 min, as tolerated. A positive result for EIB was defined as a drop in FEV1 or more than 15% within 30 min after the exercise challenged relative to the measured baseline.17 We only performed the challenge test in patients with a baseline FEV1 value that was 70% of the expected value.
In the statistical analysis, we assessed the normality of the data distribution by means of the Kolmogorov-Smirnov test. We have expressed quantitative variables as mean and standard deviation or median and interquartile range based on their distribution, and qualitative variables as absolute and relative frequencies. We compared quantitative data using the Student t test for independent samples and qualitative data using the Pearson chi square test. We assessed the diagnostic performance of the specific GINA item for detection of EIB by means of the receiver operating characteristic (ROC) curve. The statistical analysis was performed with the software SPSS version 17.0. All tests were two-tailed, and we considered differences significant if the p-value was equal or less than 0.05.
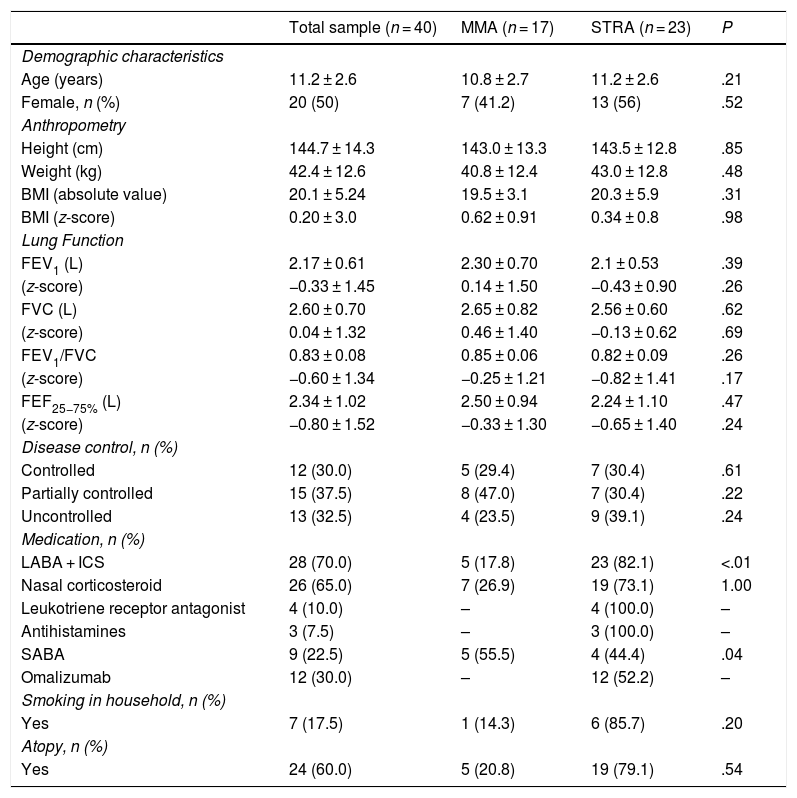
ResultsWe invited 45 patients to participate, but ended up excluding 5 because they were unable to perform the exercise challenge. Thus, the total sample consisted of 40 children and adolescents with asthma, 17 in the MMA group and 23 in the STRA group. The mean age of the overall sample was 11.2 years. Twenty participants (50%) were male, and the mean BMI z-score was 0.20. Based on the GINA questionnaire, 28 patients (70%) had uncontrolled and/or partially controlled asthma. The mean FEV1z-score was –0.33, and the mean FEV1/FVC ratio was –0.60. There were no significant differences in pulmonary function parameters between the groups. Similarly, we found no significant differences between the groups in the comparison of demographic, anthropometric, asthma control variables and other pulmonary function variables (Table 1).
Characteristics of the patients under study.
| Total sample (n = 40) | MMA (n = 17) | STRA (n = 23) | P | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years) | 11.2 ± 2.6 | 10.8 ± 2.7 | 11.2 ± 2.6 | .21 |
| Female, n (%) | 20 (50) | 7 (41.2) | 13 (56) | .52 |
| Anthropometry | ||||
| Height (cm) | 144.7 ± 14.3 | 143.0 ± 13.3 | 143.5 ± 12.8 | .85 |
| Weight (kg) | 42.4 ± 12.6 | 40.8 ± 12.4 | 43.0 ± 12.8 | .48 |
| BMI (absolute value) | 20.1 ± 5.24 | 19.5 ± 3.1 | 20.3 ± 5.9 | .31 |
| BMI (z-score) | 0.20 ± 3.0 | 0.62 ± 0.91 | 0.34 ± 0.8 | .98 |
| Lung Function | ||||
| FEV1 (L) | 2.17 ± 0.61 | 2.30 ± 0.70 | 2.1 ± 0.53 | .39 |
| (z-score) | −0.33 ± 1.45 | 0.14 ± 1.50 | −0.43 ± 0.90 | .26 |
| FVC (L) | 2.60 ± 0.70 | 2.65 ± 0.82 | 2.56 ± 0.60 | .62 |
| (z-score) | 0.04 ± 1.32 | 0.46 ± 1.40 | −0.13 ± 0.62 | .69 |
| FEV1/FVC | 0.83 ± 0.08 | 0.85 ± 0.06 | 0.82 ± 0.09 | .26 |
| (z-score) | −0.60 ± 1.34 | −0.25 ± 1.21 | −0.82 ± 1.41 | .17 |
| FEF25−75% (L) | 2.34 ± 1.02 | 2.50 ± 0.94 | 2.24 ± 1.10 | .47 |
| (z-score) | −0.80 ± 1.52 | −0.33 ± 1.30 | −0.65 ± 1.40 | .24 |
| Disease control, n (%) | ||||
| Controlled | 12 (30.0) | 5 (29.4) | 7 (30.4) | .61 |
| Partially controlled | 15 (37.5) | 8 (47.0) | 7 (30.4) | .22 |
| Uncontrolled | 13 (32.5) | 4 (23.5) | 9 (39.1) | .24 |
| Medication, n (%) | ||||
| LABA + ICS | 28 (70.0) | 5 (17.8) | 23 (82.1) | <.01 |
| Nasal corticosteroid | 26 (65.0) | 7 (26.9) | 19 (73.1) | 1.00 |
| Leukotriene receptor antagonist | 4 (10.0) | – | 4 (100.0) | – |
| Antihistamines | 3 (7.5) | – | 3 (100.0) | – |
| SABA | 9 (22.5) | 5 (55.5) | 4 (44.4) | .04 |
| Omalizumab | 12 (30.0) | – | 12 (52.2) | – |
| Smoking in household, n (%) | ||||
| Yes | 7 (17.5) | 1 (14.3) | 6 (85.7) | .20 |
| Atopy, n (%) | ||||
| Yes | 24 (60.0) | 5 (20.8) | 19 (79.1) | .54 |
Data presented as mean and standard deviation, except for sex and disease control (absolute and relative frequency). BMI, body mass index; FEV1, forced expiratory volume in one second; FEF25-75%, forced expiratory flow between 25 and 75% of vital capacity; FVC, forced vital capacity; ICS < inhaled corticosteroids; LABA, long-acting β2 agonists; MMA, mild/moderate asthma; SABA, short-acting β2 agonists; STRA, severe therapy-resistant asthma.
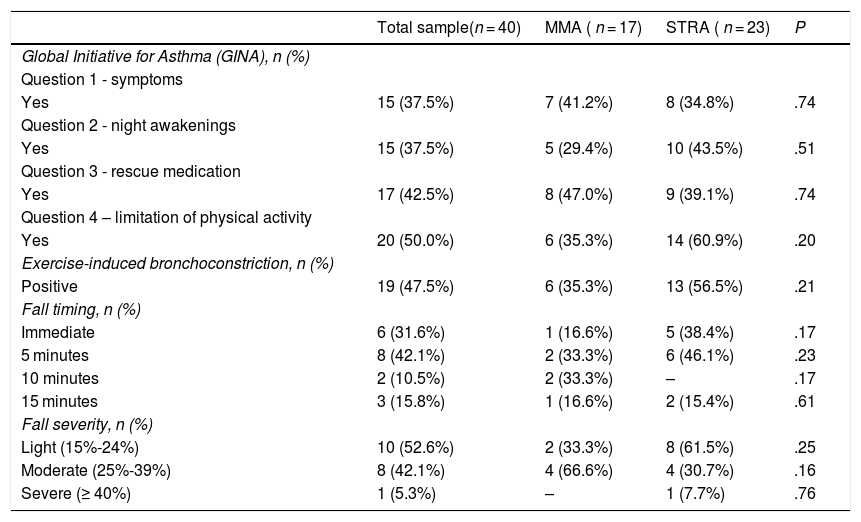
As concerns the GINA questionnaire, the percentage of positive answers ranged from 37.5% to 50% for items 1 (symptoms), 2 (night awakening) and 3 (reliever medication). Although most patients in the STRA group (60.9%) answered item 4 (physical activity) in the positive, there were no significant differences compared to the MMA group (35.3%; P = .10). Out of all the patients evaluated, 19 (47.5%) had a fall in FEV1 in the exercise challenge, 13 in the STRA group (56.5%) and 6 in the MMA group (35.2%). The decrease was predominantly observed at 5 min, and in most cases involved a mild drop in pulmonary function (of 15%-24%). We observed no differences between the groups when we compared these variables (Table 2).
Results of the GINA asthma control questionnaire and exercise-induced bronchoconstriction assessment.
| Total sample(n = 40) | MMA ( n = 17) | STRA ( n = 23) | P | |
|---|---|---|---|---|
| Global Initiative for Asthma (GINA), n (%) | ||||
| Question 1 - symptoms | ||||
| Yes | 15 (37.5%) | 7 (41.2%) | 8 (34.8%) | .74 |
| Question 2 - night awakenings | ||||
| Yes | 15 (37.5%) | 5 (29.4%) | 10 (43.5%) | .51 |
| Question 3 - rescue medication | ||||
| Yes | 17 (42.5%) | 8 (47.0%) | 9 (39.1%) | .74 |
| Question 4 – limitation of physical activity | ||||
| Yes | 20 (50.0%) | 6 (35.3%) | 14 (60.9%) | .20 |
| Exercise-induced bronchoconstriction, n (%) | ||||
| Positive | 19 (47.5%) | 6 (35.3%) | 13 (56.5%) | .21 |
| Fall timing, n (%) | ||||
| Immediate | 6 (31.6%) | 1 (16.6%) | 5 (38.4%) | .17 |
| 5 minutes | 8 (42.1%) | 2 (33.3%) | 6 (46.1%) | .23 |
| 10 minutes | 2 (10.5%) | 2 (33.3%) | – | .17 |
| 15 minutes | 3 (15.8%) | 1 (16.6%) | 2 (15.4%) | .61 |
| Fall severity, n (%) | ||||
| Light (15%-24%) | 10 (52.6%) | 2 (33.3%) | 8 (61.5%) | .25 |
| Moderate (25%-39%) | 8 (42.1%) | 4 (66.6%) | 4 (30.7%) | .16 |
| Severe (≥ 40%) | 1 (5.3%) | – | 1 (7.7%) | .76 |
MMA, mild and moderate asthma; STRA, severe therapy-resistant asthma.
Data presented as mean absolute and relative frequencies.
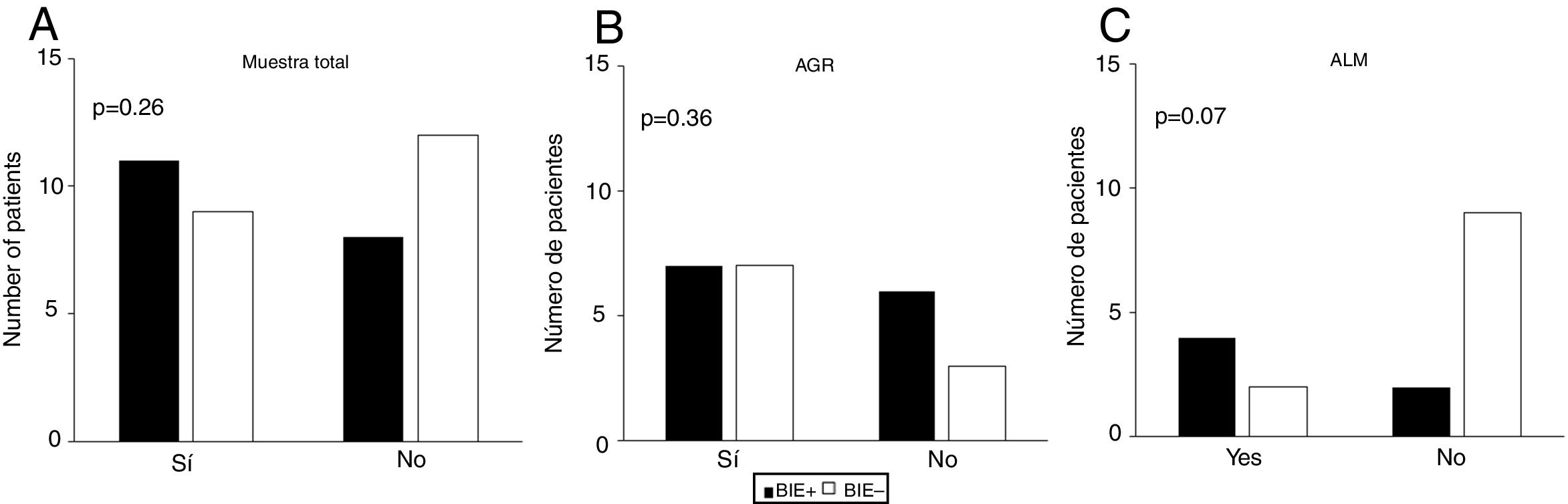
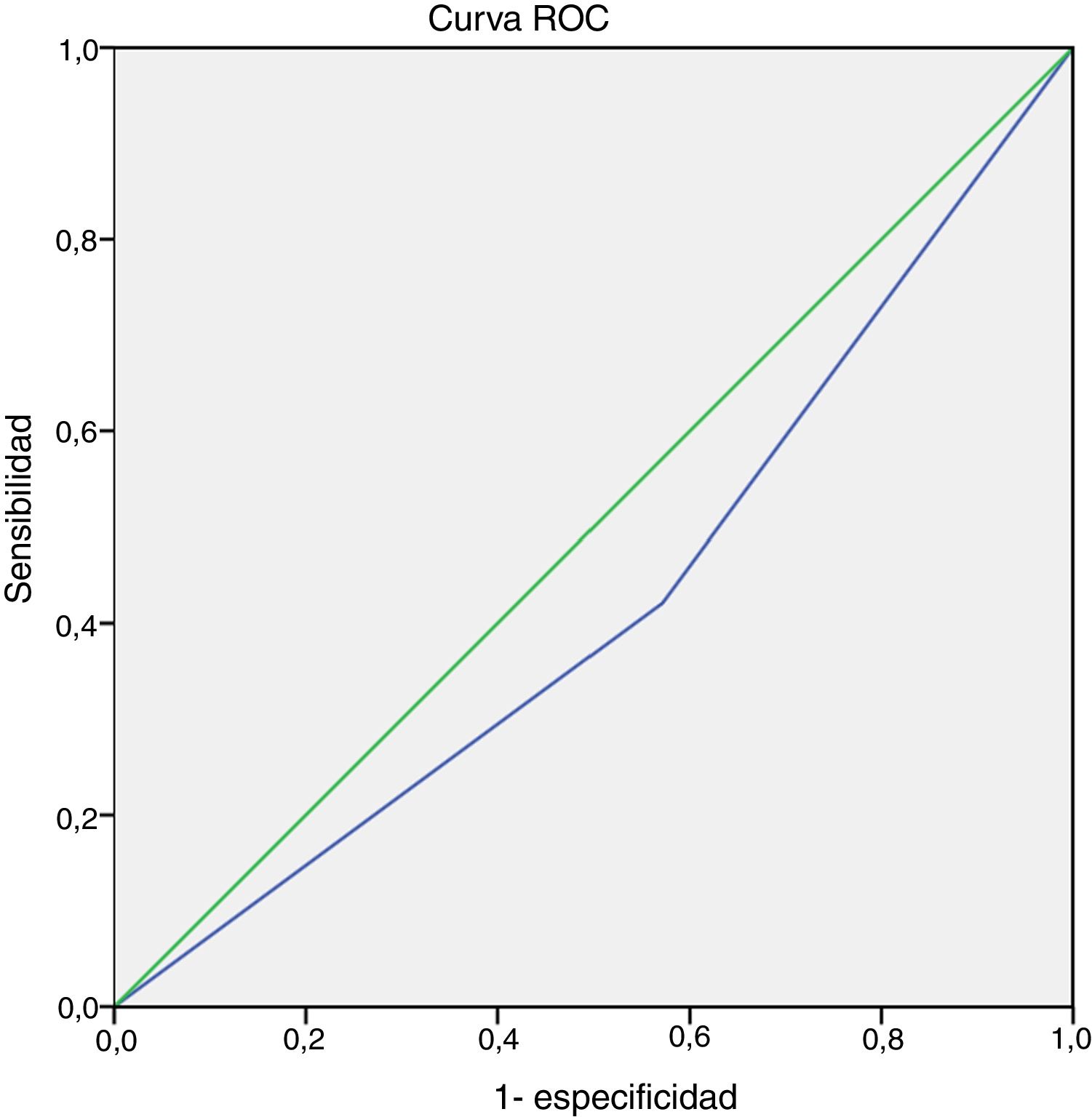
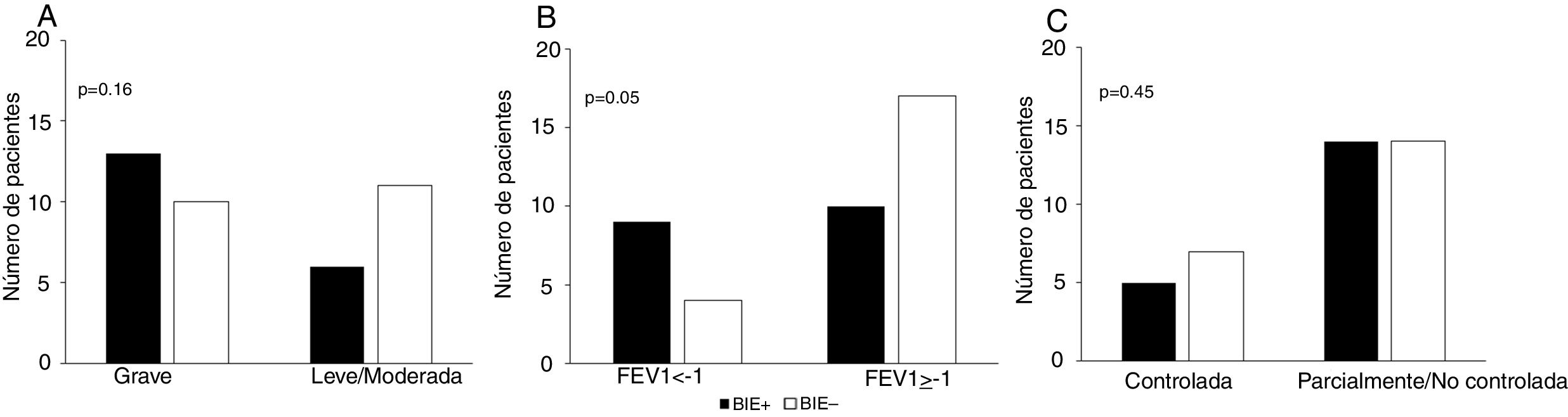
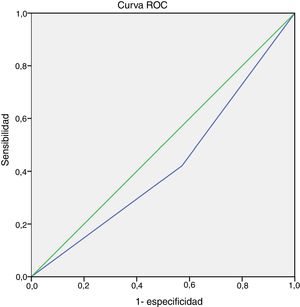
We did not observe an association between item 4 (physical activity) of the GINA questionnaire and EIB in either the total sample (P = .26) or in the MMA (P = .07) or STRA (P = .36) groups (Fig. 1). Also, we found a sensitivity of 42.10% and a specificity of 57.10% (95% confidence interval [CI], 0.24−0.60) for this item for detection of EIB in children and adolescents in the total sample (n = 40). The area under the ROC curve confirmed a low predictive power (Fig. 2). The results continued to be poor when we analysed the MMA and STRA groups separately (data not shown). We found a significantly higher frequency of EIB in participants with low FEV1 values (z-score < –1.0) compared to those with higher values (z-score ≥ –1.0) (P = .05). However, there were no significant differences in the frequency of EIB based on disease severity or disease control (Fig. 3).
Association of the answer to item 4 (physical activity) of the Global Initiative for Asthma (GINA) with exercise-induced bronchoconstriction (EIB) in (A) total sample, (B) severe therapy-resistant asthma group (STRA), and (C) mild/moderate asthma group (MMA). The p-values shown correspond to the chi square test.
Our findings demonstrate that the diagnostic power of item 4 (regarding the symptoms associated with physical activity) of the GINA questionnaire for detection of EIB in children and adolescents with MMA and STRA is inadequate. The ROC curve data and the low sensitivity and specificity evince the limitations of this tool.
Several studies have evaluated existing clinical questionnaires used for diagnosing EIB in children and adolescents, but they have found a low or moderate sensitivity and specificity.4,6,11,18,19 Most of these instruments assess the presence of the most common signs and symptoms of the disease (cough, tightness in the chest and dyspnoea) during and/or after physical activity in a given time interval prior to testing (1–12 months). Yet, from a clinical perspective, their discriminatory power seems insufficient. A poor level of fitness, obesity and sedentary life habits may be confounding factors in the interpretation of EIB symptoms in children and adolescents. To the best of our knowledge, ours is the first study to test the diagnostic performance of the GINA questionnaire (specifically, item 4) for detection of bronchial hyperresponsiveness during the exercise challenge test in asthmatic children and adolescents. Our study demonstrated that the GINA questionnaire has insufficient power to predict EIB compared to the exercise challenge, with a sensitivity of 42.1% and a specificity of 57.1%. These results are consistent with a previous study11 that found a low diagnostic accuracy (70.2% sensitivity and 49.1% specificity) of a questionnaire score for detection of EIB in a methacholine challenge test. In addition, our findings are also consistent with those of Westergren et al.,10 in whose study responses to the item ‘present feeling that asthma restrains physical activity’ were not able to accurately detect EIB. Moreover, out of 2 studies that analysed the association between the Asthma Control Test (ACT) and the exercise challenge,20,21 only one found a high specificity and both found a low sensitivity for detection of EIB in children older than 12 years.21 Thus, although some studies show that certain tests may be used as an alternative method to diagnose EIB and estimate the level of eosinophilic inflammation in the absence of a gold standard,22,23 existing clinical questionnaires continue to perform poorly in the paediatric population. It is worth noting that in our study we assessed accuracy based on the specific use of item 4 in the GINA questionnaire, so we cannot rule out the possibility that the addition of other items or a symptom questionnaire would have improved its performance. In any case, we believe that its use by physicians in clinical settings for the purpose of identifying exercise-induced symptoms or in the prescription of exercise should be avoided, and that the exercise test should be used whenever it is available and necessary.
The prevalence of EIB in the paediatric population varies widely, ranging from 20% to 90% of total cases of asthma.2 The prevalence in our study was within that range, as almost half the patients (48%) had EIB diagnosed in the exercise challenge. The variation in the prevalence of EIB is probably explained by differences between geographical regions and in population characteristics. Furthermore, environmental temperature, seasons, humidity, and other environmental factors (air pollution, allergens) may have an impact on prevalence.2 Although our study found a higher percentage of EIB in the STRA group (21.2% greater), this difference was not statistically significant.
We did not find any differences in pulmonary function and disease control between the MMA and STRA groups. Some studies have found that spirometry may not be sensitive enough for detection of pulmonary changes in some chronic respiratory diseases in children, including asthma24 and post-infectious bronchiolitis obliterans.25 Several studies have found pulmonary function values within or close to the normal range, even in patients with STRA.24,25,26 These results differ from those in adults, who seem to have greater lung function impairment.27 These findings in children may be explained, at least partially, by the shorter duration of disease, the use of new pharmacological therapies (omalizumab), and a lesser degree of bronchial remodelling. In addition, when it came to disease control, uncontrolled asthma was more frequent in the STRA group (15.6% higher), but the difference between the groups was not significant. These results may be influenced by the fact that patients are being followed up at the outpatient level with regular visits, which allows optimization of treatment.
Our findings also demonstrate that lower FEV1 baseline values (z-score < –1) are associated with a higher frequency of EIB. In adherence with international guidelines, the FEV1 is one of the most frequently used clinical parameters for diagnosis and monitoring of asthma.1 Our findings were consistent with those of previous studies,28,29 with an association of low FEV1 values with clinical worsening, exacerbation and EIB. A recent study in children and adolescents with asthma30 found a significant association between FEV1 values and asthma control (GINA), suggesting that FEV1 is a marker of exacerbation. Further data indicate that the FEV1 is associated with important clinical markers in children with asthma, including symptoms and exacerbations.28
There are limitations to our study. First, due to its cross-sectional design, the study analysed both the clinical item and the exercise challenge at a single timepoint. Asthma is known to have a changing and dynamic nature in terms of symptoms and control. A longitudinal study may provide a better understanding of this issue. Second, some patients used omalizumab (anti-IgE therapy), which may have led to higher rates of disease control and a lower prevalence of EIB in patients with STRA. However, given the benefits omalizumab offers to patients with STRA,30 discontinuation of the drug during the period under study would have been inappropriate.
In conclusion, our results demonstrate that the GINA question about physical activity has a poor diagnostic power for detection of EIB in children and adolescents with asthma, regardless of severity. Therefore, the indication for the exercise challenge test for diagnosis of EIB should be further discussed in guidelines and recommendations with the aim of preventing frequent false positives or false negatives in the paediatric population.
FundingThis work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – funding code 001 - and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Please cite this article as: Schiwe D, Heinzmann-Filho JP, Schindel CS, Gheller MF, Campos NE, Santos G et al. Rendimiento diagnóstico de la pregunta concerniente a la actividad física del cuestionario GINA para la detección de asma y broncoconstricción inducidas por el ejercicio. An Pediatr (Barc). 2021;95:40–47.