Diagnostic delay of inflammatory bowel disease in children might be responsible for complications and a poor response to treatment. The study of diagnostic delay and its determining factors may help implement corrective measures and improve the prognosis of the disease.
Patients and methodsA retrospective study of the information collected from primary care medical records and that from the paediatric gastroenterology service at a tertiary hospital between 2000 and 2012 was carried out in 53 patients: 31 with Crohn's disease, 19 with ulcerative colitis, and 3 with unclassified paediatric inflammatory bowel disease. The main response variable was the interval from the first physician–patient contact to diagnosis.
ResultsThe median time to diagnosis was 12 weeks (interquartile range 5–24). However for 26.3% of the ulcerative colitis cases and 25.8% of the Crohn's disease cases, the interval was longer than 1 year. There was a more marked delay trend in Crohn's disease cases, but it was not statistically significant. None of the evaluated risk factors were associated with a relevant diagnostic delay, although it tended to be longer in younger children.
ConclusionsWhereas the median delay for paediatric inflammatory bowel disease seems to be acceptable, the diagnostic time spans are considerable for a large proportion of children with heterogeneous clinical characteristics. Further research into lost diagnostic opportunities needs to be carried out.
El retraso diagnóstico (RD) de la enfermedad inflamatoria intestinal pediátrica (EII-P) puede conllevar la aparición de complicaciones y una menor respuesta al tratamiento. Estudiar el RD y los factores que lo condicionan ayudaría a implementar medidas correctoras y mejorar la evolución de la enfermedad.
Pacientes y métodosUn total de 53 casos (31 de enfermedad de Crohn [EC], 19 de colitis ulcerosa [CU] y 3 EII-P no clasificadas) entre 2000 y 2012 se evaluaron de forma retrospectiva a través de la información recogida en las historias clínicas de atención primaria y las de un Servicio de Gastroenterología infantil de un hospital terciario. La variable respuesta principal fue el tiempo entre el primer contacto médico-paciente y el diagnóstico.
ResultadosEl tiempo mediano de RD fue de 12 semanas (rango intercuartílico 5-24). Sin embargo, un 26,3% de las CU y un 25,8% de las EC presentaron un RD superior a un año. Ninguno de los factores de riesgo estudiados se asoció significativamente a un RD relevante pero los niños de menor edad presentaron una tendencia a un mayor RD.
ConclusionesAunque el RD mediano de la EII-P parece aceptable, existe una proporción importante de niños con unas características clínicas heterogéneas y unos tiempos diagnósticos considerables. Se debería profundizar en el análisis de las oportunidades perdidas de diagnóstico.
The onset of inflammatory bowel disease (IBD) usually occurs at around 20–30 years of age, and is therefore considered a young adult disease. However, there is a subset of patients in whom onset occurs during childhood that, depending on the published series, amounts to 20–25% of the total.1
In the past 20 years there has been an increase in the incidence of Crohn's disease (CD), while the number of ulcerative colitis (UC) diagnoses has remained stable or declined. This phenomenon seems to apply to childhood cases as well.2 The diagnosis of IBD, especially in this age group, may be a considerable challenge due to the symptom overlap with functional bowel disorders and its variable clinical expression.
It has been described that isolated ileal involvement in CD and consumption of anti-inflammatory agents and male sex in UC are risk factors for diagnostic delay (DD).3 There is little evidence on the patient characteristics that contribute to a longer DD in children with IBD. Some analyses based on European registries concluded that younger children and children with ileal CD experience the longest delays in diagnosis.4
IBD comprises a group of chronic and incurable diseases, and there is evidence that delays in the initiation of treatment are associated with poorer responses to it and with a more severe course of disease.5–8 Nearly 20% of patients younger than 16 years diagnosed with CD have growth delay or a short stature. The proportion in UC patients ranges from 5% to 10%.9 Children may catch up in weight and height with pharmacological and nutritional treatment, but there is also evidence that a prolonged DD influences the final height of children with CD and facilitates the proximal extension of UC.10 Furthermore, the diagnosis of IBD in a child or adolescent has a negative impact not only on physical aspects, but also has a negative psychological, familial and social impact.11
For the above reasons, early diagnosis is considered a crucial element in the appropriate management of paediatric IBD. Our aim was to study the duration of DD in patients in our setting in order to describe its main stages, identify the main factors at play, and propose corrective measures.
Patients and methodsWe conducted a retrospective study with the main objective of describing DD in paediatric IBD in our setting. We set out to determine the time elapsed between the main care milestones: T1, time elapsed between the onset of symptoms and the first medical visit; T2, time elapsed between referral to the hospital and the first appointment with a specialist in paediatric gastroenterology; T3, time elapsed between the first hospital visit and the first digestive endoscopy; and T4, time elapsed between the first medical visit and the explicit documentation of the diagnosis in the medical records based on the clinical presentation, laboratory data, digestive endoscopy and histopathology. We defined DD as equivalent to T4 and considered that DD was relevant for T4 values above the 75th percentile of each diagnostic group (UC, CD, and unspecified IBD [IBD-U]).
The secondary objectives were to identify the work setting and degree of specialisation of the first doctor seen by the patient and of the doctor that made the referral to the specialist, as well as the risk factors for having a relevant DD. To this end, we collected data pertaining to the clinical presentation, use of diagnostic tools, and extent of disease at the time of diagnosis according to the new Paris classification.12
Any enthesitis and arthritis suspected to be an extraintestinal manifestation of IBD was confirmed by a paediatric rheumatologist. The severity of disease flares was evaluated by means of the Paediatric Ulcerative Colitis Activity Index (PUCAI)13 and the Paediatric Crohn's Disease Activity Index (PCDAI).14
We identified patients younger than 18 years diagnosed with IBD from the database of the clinical documentation department of our hospital. The main sources of data were the paper medical records, supplemented by electronic record files in both our hospital's database, set up in 2010, and the primary care (PC) system database, which allowed for a better description of the time preceding the first contact at the hospital.
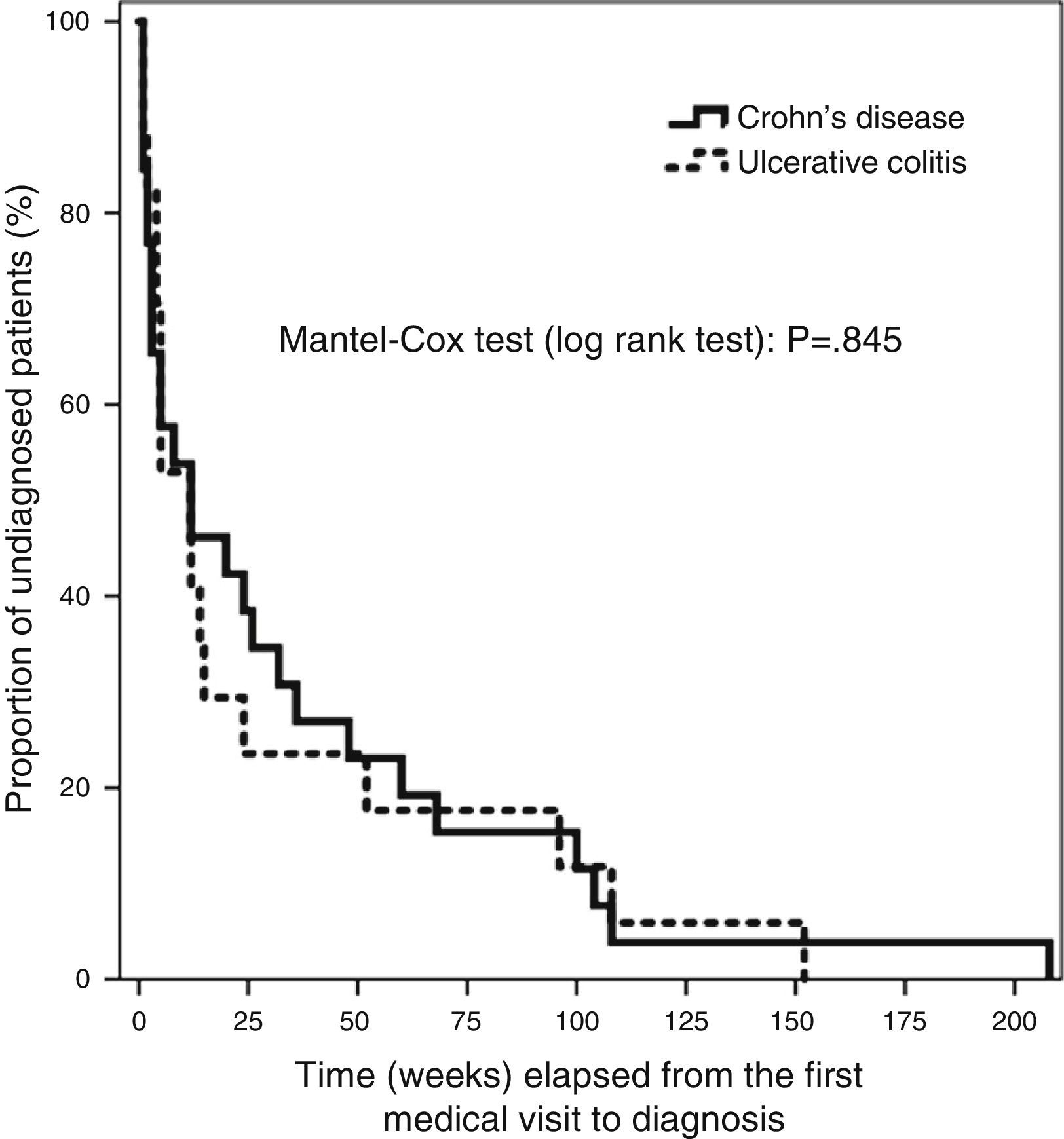
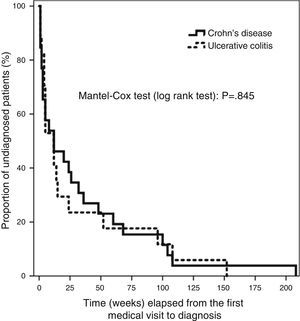
We entered the data in a database created specifically for the purpose (Microsoft Access 2010). The statistical analysis was performed with the SPSS 19.0 software. Qualitative data were expressed as frequencies and percentages. The Kolmogorov–Smirnov test revealed a non-normal distribution of the quantitative variables under study, so we summarised these data using the median and the 95% confidence interval (CI), the interquartile range (IQR) and the minimum and maximum values. We used the χ2 test to analyse the association between qualitative variables and the Mann–Whitney U nonparametric test to compare quantitative data, with the main purpose of finding the differences between the CD and UC subgroups. We used the Bonferroni post hoc test for the multiple univariate ANOVA in our assessment of risk factors. The distribution of T4 for the whole sample and for CD and UC patients separately had a skewed shape with a marked right tail, which we modified by logarithmic transformation. This allowed for the use of multiple linear regression methods in our search for factors contributing to DD. The Kaplan–Meier analysis of the main variable is shown in Fig. 1, and the differences in elapsed time by type of IBD were assessed by means of the Mantel–Cox test (log rank), based on the χ2 for equivalence of the survival distributions. We set the level of statistical significance at P<.05.
ResultsWe found data for 53 patients between January 2000 and January 2012. The cohort included 31 children with CD (58.5%), 19 with a final diagnosis of UC (35.8%) and 6 with IBD-U at the beginning of the inclusion period, 3 of which were later diagnosed as UC during the follow-up and included in the UC group for the statistical analysis.
The median age at diagnosis was 10.75 years for CD, 7.75 years for UC, and 8.83 years for IBD-U. We found a significantly higher percentage of male patients among CD cases (83.9%) compared to UC cases (47.4%) and found no differences in the home setting of the children (rural vs urban) or the prevalence of smoking at the home. We did find that family histories of IBD were more common in UC patients than in CD patients (26.3% vs 13.3%).
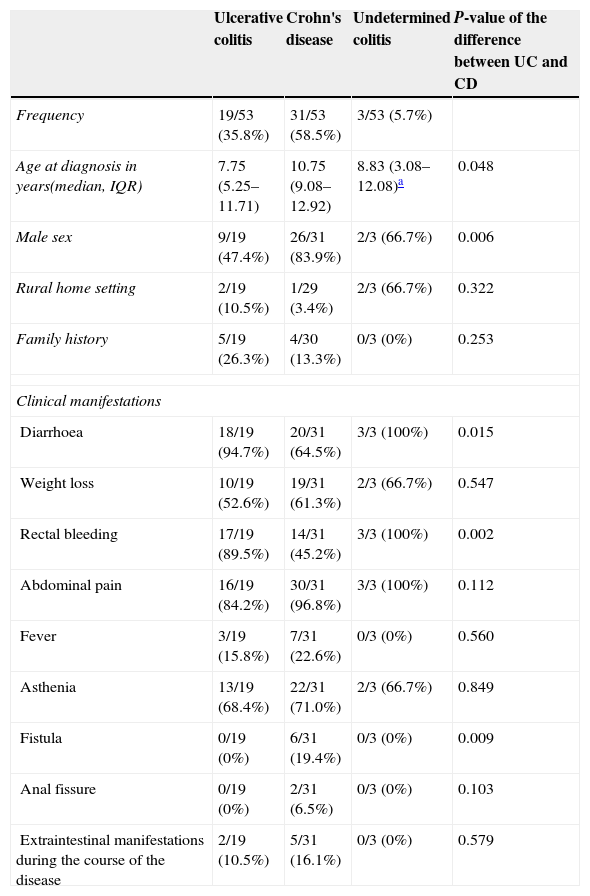
Table 1 summarises the symptoms at the onset of disease. We must note that all patients had intestinal symptoms at diagnosis (diarrhoea, rectal bleeding or abdominal pain), while extraintestinal manifestations were present in seven (13.2%): one patient with CU had erythema nodosum and another sclerosing cholangitis, and five CD patients had arthritis/enthesitis. Constitutional symptoms such as weight loss, fever and asthenia occurred more frequently in CD versus UC patients (a difference that did not reach statistical significance) and always in combination with digestive symptoms. Only one patient with CD had isolated abdominal pain at the onset. None of the children exhibited growth delay as the sole clinical manifestation at the onset.
Demographic data and clinical manifestations at onset.
| Ulcerative colitis | Crohn's disease | Undetermined colitis | P-value of the difference between UC and CD | |
|---|---|---|---|---|
| Frequency | 19/53 (35.8%) | 31/53 (58.5%) | 3/53 (5.7%) | |
| Age at diagnosis in years(median, IQR) | 7.75 (5.25–11.71) | 10.75 (9.08–12.92) | 8.83 (3.08–12.08)a | 0.048 |
| Male sex | 9/19 (47.4%) | 26/31 (83.9%) | 2/3 (66.7%) | 0.006 |
| Rural home setting | 2/19 (10.5%) | 1/29 (3.4%) | 2/3 (66.7%) | 0.322 |
| Family history | 5/19 (26.3%) | 4/30 (13.3%) | 0/3 (0%) | 0.253 |
| Clinical manifestations | ||||
| Diarrhoea | 18/19 (94.7%) | 20/31 (64.5%) | 3/3 (100%) | 0.015 |
| Weight loss | 10/19 (52.6%) | 19/31 (61.3%) | 2/3 (66.7%) | 0.547 |
| Rectal bleeding | 17/19 (89.5%) | 14/31 (45.2%) | 3/3 (100%) | 0.002 |
| Abdominal pain | 16/19 (84.2%) | 30/31 (96.8%) | 3/3 (100%) | 0.112 |
| Fever | 3/19 (15.8%) | 7/31 (22.6%) | 0/3 (0%) | 0.560 |
| Asthenia | 13/19 (68.4%) | 22/31 (71.0%) | 2/3 (66.7%) | 0.849 |
| Fistula | 0/19 (0%) | 6/31 (19.4%) | 0/3 (0%) | 0.009 |
| Anal fissure | 0/19 (0%) | 2/31 (6.5%) | 0/3 (0%) | 0.103 |
| Extraintestinal manifestations during the course of the disease | 2/19 (10.5%) | 5/31 (16.1%) | 0/3 (0%) | 0.579 |
CD: Crohn's disease; UC: ulcerative colitis.
Seven cases were diagnosed before 5 years of age (13.2%: 4 with UC, 2 with CD and 1 with IBD-U) and all had onset with diarrhoea and abdominal pain. When we stratified by age and analysed CD and UC separately we did not find significant differences in the initial symptoms, although we observed a trend towards greater growth delay in children younger than 5 years.
A colonoscopy was performed in every child in the cohort. Due to technical limitations, ileoscopies were performed satisfactorily in only 58.1% of CD patients. None of the UC patients were considered for an upper GI endoscopy, but a barium enema was performed in 36.8% of these patients at some point during their follow-up, mostly due to an incomplete colonoscopy during the diagnostic process. Magnetic resonance enterography was performed in 74.2% of CD patients, especially in cases diagnosed in later years, compared to 15.8% of UC patients. Examination by capsule endoscopy was also more frequent in CD patients (61.3%). Only one patient that was finally diagnosed with UC underwent this procedure while still diagnosed with IBD-U.
Table 2 shows the distribution of UC and CD cases into the subgroups defined by the new Paris classification. We were able to retrieve information on the extent of the disease for every patient with CD and UC. We only lacked sufficient data to determine the severity of the first flare of the disease for two patients with UC that had been transferred from other hospitals.
Severity and extent of disease at the time of diagnosis as defined by the Paris classification.
| Crohn's disease | |
|---|---|
| Age | |
| A1a, younger than 10 years | 13/31 (41.9%) |
| A1b, between 10 and 17 years | 18/31 (58.1%) |
| A2, older than 17 years | 0/31 (0%) |
| Extent | |
| L1, ileal or limited caecal | 11/31 (35.5%) |
| L2, colonic | 4/31 (12.9%) |
| L3, ileocolonic | 10/31 (32.3%) |
| L1+L4a, upper disease proximal to ligament of Treitz | 1/31 (3.2%) |
| L1+L4b, upper disease distal to ligament of Treitz and proximal to distal 1/3 ileum | 5/31 (16.1%) |
| Behaviour | |
| B1, inflammatory nonstricturing nonpenetrating | 16/31 (51.6%) |
| B2, stricturing | 9/31 (29%) |
| B2p, stricturing with perianal involvement | 2/31 (6.5%) |
| B3, penetrating | 1/31 (3.2%) |
| B3p, penetrating with perianal involvement | 2/31 (6.5%) |
| B2B3, stricturing-penetrating | 3/31 (9.7%) |
| Growth | |
| G0, no evidence of growth delay | 17/31 (54.8%) |
| G1, growth delay | 14/31 (45.2%) |
| Ulcerative colitis | |
|---|---|
| Extent | |
| E1, proctitis | 1/19 (5.3%) |
| E2, left sided UC (distal to splenic flexure) | 4/19 (21.1%) |
| E3, extensive colitis (distal to hepatic flexure) | 1/19 (5.3%) |
| E4, pancolitis (proximal to hepatic flexure) | 13/19 (68.4%) |
| Severity | |
| S0, never severe | 11/17 (64.7%) |
| S1, ever severe | 6/17 (35.3%) |
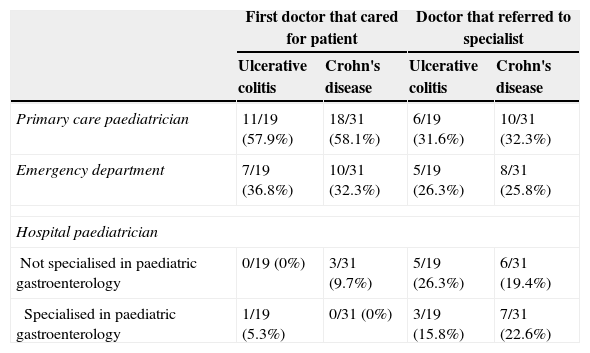
The number of visits made to the emergency department for clinical features attributable to IBD before diagnosis was low, with a median of one visit for both CD and UC patients. However, 71.4% of the children were seen at least once in hospital emergency departments, which resulted in 26% of the referrals to the paediatric gastroenterology department. Most referrals to the specialist were made from PC (Table 3). A gastroenterologist was the first doctor to see the patient in only one case. While only 6% of the patients were first seen by hospital paediatricians that did not specialise in gastroenterology, these providers made the referral to the specialist in 22% of the cases.
Doctors involved in the diagnosis.
| First doctor that cared for patient | Doctor that referred to specialist | |||
|---|---|---|---|---|
| Ulcerative colitis | Crohn's disease | Ulcerative colitis | Crohn's disease | |
| Primary care paediatrician | 11/19 (57.9%) | 18/31 (58.1%) | 6/19 (31.6%) | 10/31 (32.3%) |
| Emergency department | 7/19 (36.8%) | 10/31 (32.3%) | 5/19 (26.3%) | 8/31 (25.8%) |
| Hospital paediatrician | ||||
| Not specialised in paediatric gastroenterology | 0/19 (0%) | 3/31 (9.7%) | 5/19 (26.3%) | 6/31 (19.4%) |
| Specialised in paediatric gastroenterology | 1/19 (5.3%) | 0/31 (0%) | 3/19 (15.8%) | 7/31 (22.6%) |
Children who first sought care for their IBD symptoms in a PC setting experienced a longer DD than children who were initially assessed in a hospital emergency department, with a median DD of 26 weeks (IQR, 11.5–78) versus 3 weeks (IQR, 1.5–5). However, patients first seen in PC had delayed growth less frequently (37.5% vs 69%), clinical features that were less specific, and higher T2 values. Thus, if the patient was referred from PC, the median time to referral was 2 weeks, with an IQR of 1–4 weeks and a maximum of 10 weeks, while referrals from the emergency department (in all cases, from our hospital) never took longer than one week. The distribution by age was similar in both groups (RIQ of 7.5–11.7 years for PC and 8.6–11.4 years for the emergency department).
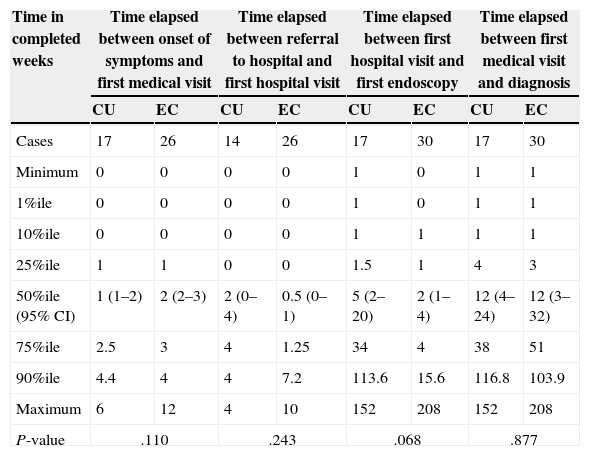
The median time elapsed from the initial symptoms of IBD to the first visit to a doctor was 2 weeks (95% CI, 1–2; RIQ, 1–3 weeks). There was a median delay of 12 weeks between this first visit and the definitive diagnosis (95% CI, 5–24 weeks; RIQ, 3–48 weeks). The rest of the time intervals is presented in Table 4. We did not find differences in any of the 4 time intervals studied when we analysed UC and CD separately. There was no difference in DD between UC and CD patients when we applied survival analysis methods (Fig. 1). We considered DD relevant if it exceeded 38 weeks in UC patients and starting at 51 weeks in CD patients. Delays longer than one year occurred in 26.3% of UC patients and 25.8% of CD patients.
Distribution of time to diagnosis for ulcerative colitis and Crohn's disease.
| Time in completed weeks | Time elapsed between onset of symptoms and first medical visit | Time elapsed between referral to hospital and first hospital visit | Time elapsed between first hospital visit and first endoscopy | Time elapsed between first medical visit and diagnosis | ||||
|---|---|---|---|---|---|---|---|---|
| CU | EC | CU | EC | CU | EC | CU | EC | |
| Cases | 17 | 26 | 14 | 26 | 17 | 30 | 17 | 30 |
| Minimum | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| 1%ile | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| 10%ile | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| 25%ile | 1 | 1 | 0 | 0 | 1.5 | 1 | 4 | 3 |
| 50%ile (95% CI) | 1 (1–2) | 2 (2–3) | 2 (0–4) | 0.5 (0–1) | 5 (2–20) | 2 (1–4) | 12 (4–24) | 12 (3–32) |
| 75%ile | 2.5 | 3 | 4 | 1.25 | 34 | 4 | 38 | 51 |
| 90%ile | 4.4 | 4 | 4 | 7.2 | 113.6 | 15.6 | 116.8 | 103.9 |
| Maximum | 6 | 12 | 4 | 10 | 152 | 208 | 152 | 208 |
| P-value | .110 | .243 | .068 | .877 | ||||
CD: Crohn's disease; UC: ulcerative colitis.
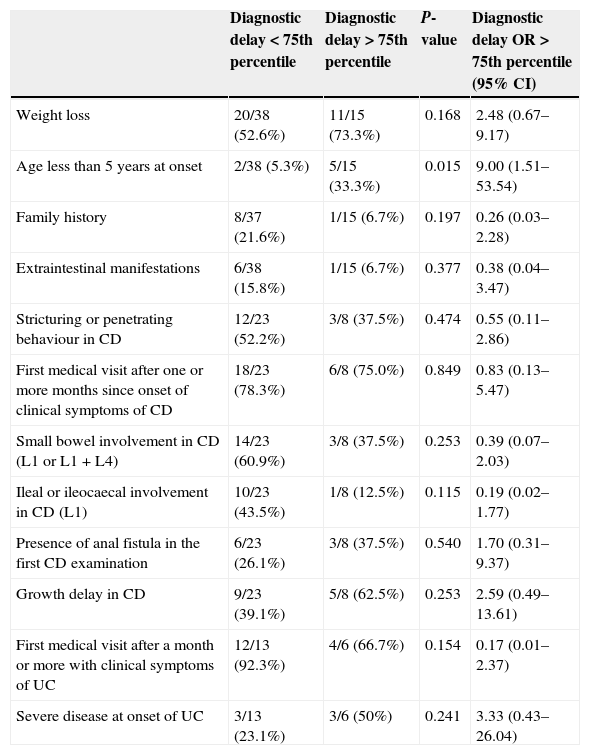
The univariate analysis (Table 5) revealed that age below 5 years was the only factor with a significant influence on DD in IBD-U. At any rate, the significance of this finding disappeared once the Bonferroni corrections were applied. The multiple linear regression analysis did not identify any variables significantly associated with the duration of DD nor a satisfactory model for its prediction. We also found no associations when we used the median T4 as a measure of the DD.
Univariate analysis. Clinical features at diagnosis: impact on diagnostic delay.
| Diagnostic delay<75th percentile | Diagnostic delay>75th percentile | P-value | Diagnostic delay OR>75th percentile (95% CI) | |
|---|---|---|---|---|
| Weight loss | 20/38 (52.6%) | 11/15 (73.3%) | 0.168 | 2.48 (0.67–9.17) |
| Age less than 5 years at onset | 2/38 (5.3%) | 5/15 (33.3%) | 0.015 | 9.00 (1.51–53.54) |
| Family history | 8/37 (21.6%) | 1/15 (6.7%) | 0.197 | 0.26 (0.03–2.28) |
| Extraintestinal manifestations | 6/38 (15.8%) | 1/15 (6.7%) | 0.377 | 0.38 (0.04–3.47) |
| Stricturing or penetrating behaviour in CD | 12/23 (52.2%) | 3/8 (37.5%) | 0.474 | 0.55 (0.11–2.86) |
| First medical visit after one or more months since onset of clinical symptoms of CD | 18/23 (78.3%) | 6/8 (75.0%) | 0.849 | 0.83 (0.13–5.47) |
| Small bowel involvement in CD (L1 or L1 + L4) | 14/23 (60.9%) | 3/8 (37.5%) | 0.253 | 0.39 (0.07–2.03) |
| Ileal or ileocaecal involvement in CD (L1) | 10/23 (43.5%) | 1/8 (12.5%) | 0.115 | 0.19 (0.02–1.77) |
| Presence of anal fistula in the first CD examination | 6/23 (26.1%) | 3/8 (37.5%) | 0.540 | 1.70 (0.31–9.37) |
| Growth delay in CD | 9/23 (39.1%) | 5/8 (62.5%) | 0.253 | 2.59 (0.49–13.61) |
| First medical visit after a month or more with clinical symptoms of UC | 12/13 (92.3%) | 4/6 (66.7%) | 0.154 | 0.17 (0.01–2.37) |
| Severe disease at onset of UC | 3/13 (23.1%) | 3/6 (50%) | 0.241 | 3.33 (0.43–26.04) |
CD: Crohn's disease; CI: confidence interval; OR: odds ratio; UC: ulcerative colitis.
We present a series of 53 patients with characteristics similar to those described in the literature for IBD-U.15 We found a proportion of male CD patients (83.9%) that was above what was previously described in the literature.16 Our results corroborate that abdominal pain is the typical presentation in paediatric patients, unlike in adults, in whom isolated diarrhoea is more frequent.16 Growth delay, reflected by a one- or two-standard deviation drop relative to the previous stature, was observed in 45.2% of CD patients before the IBD diagnosis. The cause of growth failure is multifactorial and is closely associated with the disease's inflammatory activity, which would explain the higher probability of finding it in cases with longer DDs.17 This is supported by the greater frequency of growth failure found in our population among children diagnosed before 5 years of age. Considering the problems that derive from this complication, including its psychosocial impact, the possibility of specific hormonal treatment should be evaluated by a paediatric endocrinologist in adherence with the pertinent recommendations.18
A positive family history of IBD was found more frequently in UC patients than in CD patients, and only one of the nine patients with affected first-degree relatives had a relevant DD, which suggests that a positive family history may increase the degree of suspicion for IBD.
The trend towards greater growth delay in cases with relevant DDs suggests that early diagnosis is crucial in children with IBD. Growth delay affected 50% of children with CD who were diagnosed after more than 6 months.
Endoscopy is the gold standard for the initial examination of patients with suspected IBD according to the protocol established by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition.19 The Porto criteria established that in addition to an ileonoscopy, an upper gastrointestinal endoscopy (UGIE) and multiple biopsies should be performed to determine the extent of the disease more precisely and to minimise the possibility of a IBD-U diagnosis.20 Despite having performed an UGIE in only 5 patients, our proportion of IBD-U is not similar to the figures reported in the literature.21 The main limitation of our study was the incomplete diagnosis according to the Porto criteria, which may have led to a bias in the subclassification of the extent and behaviour of the disease, and therefore in the assessment of risk factors for DD.
Previous studies have shown a high variation in the duration of DD, which is usually longer than the delay found in our study. The EPIMAD registry reports time elapsed between onset of symptoms and diagnosis of 2 months for UC and 4 months for CD in some regions in France.2 The DD was greater than 4 months in a prospective population study in Norway.22 A study of British children with IBD reported mean DDs of 4 (UC) and 5 months (CD).23 The data of the national Italian registry of paediatric IBD show DDs of 6 months for UC and 10 months for CD.24 The CEDATA registry in Germany and Austria, which collected retrospective and prospective data on 2604 children from before 1995 through August 2009, found a decreasing trend in DD in recent years, with the median DD declining to 2.5 months between 2008 and 2009.4 This epidemiological study concluded that DD was greater in younger patients and in patients with small bowel CD. These data are consistent with those of other studies that were not specifically designed to assess DD.25
The median DD in children with IBD is 12 weeks. The mean DD, on the other hand, is 31.6 weeks, which shows the influence of extremely large values in the distribution.
In CD patients, stricturing or penetrating behaviour and large bowel involvement are associated to shorter DDs.4 While our data also showed a trend towards a shorter DD in those conditions, the finding was not statistically significant. Our series included 51.6% of purely inflammatory forms of the disease, compared to the 80–90% reported in other studies.26 The greater proportion of more severe forms of the disease may have resulted in more noticeable clinical features and a shorter DD.
Another factor that may have contributed to the shorter DD in our study is that the median age of our patients was slightly higher than the median ages in other series,1,4 which may lead to an earlier presumptive diagnosis. Thus, there was a trend towards a greater prevalence of relevant DD in children younger than 5 years compared to older children, which was possibly due to a lesser degree of suspicion and less specific symptoms in the younger group.
One possible explanation for the fact that 26% of the patients referred to the specialist were referred from the hospital emergency department is that there would be a higher degree of suspicion in this setting because these cases would presumably be more severe, or because the department has a closer association with the paediatric gastroenterology specialist. On the other hand, nearly three-quarters of the patients sought emergency care in a hospital at some point, which presented the opportunity of referring this majority of patients to a specialist if it had not already been done by the healthcare centre.
A multicentre study based on the CEDATA registry demonstrated that the referring centre had an effect on the duration of DD.4 This finding seems to support that DD is not only determined by the characteristics of the patient, but also by factors related to the doctor providing care and the setting or conditions in which the doctor works. In an adult case series, the DD attributed to doctor-related factors was more pronounced in cases of CD than of UC3; however, our study showed a similar effect in both diseases. A greater degree of suspicion of CD in our setting may account for this finding. Thus, the time elapsed before the first medical visit seems to be determined by the clinical and social characteristics of the patient, and factors relating to health providers seem to be at play in subsequent time intervals.
The information contained in the medical records sufficed to conduct this study, although its retrospective design may have influenced the correct determination of the interval limits, especially the beginning of T4, that is, the time elapsed between the first doctor–patient contact and the suspicion of IBD. Furthermore, the sample size has limited the possibility of identifying risk profiles for relevant DD.
To conclude, the duration of DD in our series was slightly shorter than those reported in previous studies, which we attribute to our sample being more recent and to the likelihood of a greater knowledge of IBD in the PC and hospital emergency departments. Nevertheless, the fact that there were a large number of children with DDs exceeding one year shows that there is still room for improvement in the time intervals that depend on healthcare providers.
A method similar to the one applied in a recent study of colorectal cancer in adults27 could help identify missed chances for diagnosis. A prospective multicentre observational study similar to the ongoing study of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (Spanish Society of Paediatric Gastroenterology, Hepatology and Nutrition) could be the most suitable design to identify at-risk patients, obtain a more accurate description of DD in a larger population, and achieve our ultimate goal: the implementation of corrective measures and improvement in disease outcomes.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Arcos-Machancoses JV, Donat-Aliaga E, Polo-Miquel B, Masip-Simó E, Ribes-Koninckx C, Pereda-Pérez A. Retraso diagnóstico en la enfermedad inflamatoria intestinal pediátrica. Descripción y estudio de los factores de riesgo. An Pediatr (Barc). 2015;82:247–254.