Primary objective, to describe the management and monitorization of critically ill pediatric hemato-oncology patient (CIPHO) in the Spanish pediatric intensive care units (PICU). Secondary objective, through a literature review, to identify possible areas of improvement.
Material and methodsObservational transversal descriptive study. An anonymous web-based survey was sent to 324 Spanish pediatric intensivists from April 2011 to May 2011. None of them were pediatric residents.
ResultsThe survey was answered by 105 intensivists, 59/105 always agreed on their treatment with the oncologist. In case of hemodynamic instability, non-invasive blood pressure monitoring is always done by 85/105 and almost always optimized by intra-arterial measuring (85/105) and central venous pressure (70/105). If respiratory failure the use of non-invasive ventilation (NIPPV) is always (36/105) or frequently (60/105) established prior to conventional mechanical ventilation. To replace or withdraw non-invasive ventilation only 44/96 of the respondents to this question use a clinical protocol. Before the instauration of conventional mechanical ventilation the oncological prognosis is considered by 72/105. In case of acute oliguric renal failure the renal replacement techniques are widely used (74/105). The withdrawal of sustaining life support is frequently discussed (75/103) and agreed with the oncologist (91/103) and caregivers (81/103).
ConclusionsIn our study, despite there not being a defined standard-of-care, the respondents showed similar therapeutic and monitorization choices. The use of NIPPV as first respiratory assistance is extended. Prospective, observational and multicenter studies should be developed to establish the results of this management in this population.
Objetivo primario, definir el tratamiento y la monitorización del niño crítico con enfermedad hemato-oncológica en las unidades de cuidados intensivos españolas. El objetivo secundario fue, tras una revisión de la literatura, contextualizar el enfoque obtenido y detectar posibles puntos de mejora.
Material y métodosEstudio observacional, descriptivo y transversal. Se envió en el periodo abril del 2011-mayo del 2011 una encuesta online a 324 intensivistas y adjuntos de pediatría registrados en la Sociedad Española de Cuidados Intensivos Pediátricos.
ResultadosSe obtienen 105 respuestas globales, 59/105 indicaron acordar el tratamiento con el oncólogo. Ante hipotensión, taquicardia y requerimiento de inotrópicos, 85/105 realizan siempre monitorización no invasiva de presión arterial asociando además medición intraarterial (85/105) y casi siempre presión venosa central (70/105). Ante dificultad respiratoria, se instaura siempre (36/105) o frecuentemente (60/105) ventilación no invasiva. De forma previa a iniciar ventilación mecánica convencional, 72/105 consideran el pronóstico global del paciente. Ante fallo renal agudo oligúrico, las técnicas de depuración extrarrenal son ampliamente utilizadas (74/105). En caso de mal pronóstico, la adecuación del tratamiento es considerada de forma frecuente (75/103) y conjunta con el oncólogo (91/103) y la familia (81/103).
ConclusionesSe observa gran similitud en las respuestas a pesar de que el manejo de este tipo de pacientes no está estandarizado. En caso de dificultad respiratoria, el uso de ventilación no invasiva como primera asistencia está ampliamente extendido. El desarrollo de futuros estudios observacionales prospectivos y multicéntricos permitiría conocer los resultados derivados de este enfoque.
Nowadays there isn’t any standard-of-care of critically ill pediatric hemato-oncology patient (CIPHOP).1,2 In case of pediatric intensive care unit (PICU) admission the treatment and monitoring protocols applied are not different from those used in children without an oncological disease.3
Critically ill pediatric hemato-oncology patient survival has improved in recent years4 thanks to a better management of their complications and the evolution and development of new therapies.1,2,4 Unfortunately, the PICU mortality and morbidity are still important and discussions about which is the better therapeutic option in each moment, or when to withdraw the therapies initiated are common.5
There are nearly 1100 new cases of pediatric cancer each year in Spain.6 There are no institutions exclusively dedicated to the pediatric hemato-oncology patient (PHOP). These patients are treated in 47 hospitals with different characteristics and equipment.3,6 This heterogeneity, despite the 80% of global survival, maybe implies an obstacle to homogenize and optimize the therapies applied. The aim of this study is to describe the management and monitorization of CIPHO in the Spanish PICUs. Later, through a short literature review, a discussion about the questions obtained is done.
MethodsTransversal, observational and descriptive study performed by a web-based survey (GoogleDoc® formulary) sent to the 324 Spanish pediatric intensivists registered in the Spanish Society of Pediatric Critical Care. No pediatric residents were included in the study. The survey, detailed in Table 1, was anonymous. It was sent to intensivists regardless of the complexity of their units and patients. The intensivists were reminded of the survey every two weeks from April 2011 to May 2011. In case of no answer the participant was not considered for the final analysis. A descriptive analysis of the answers was performed using SPSS 16.0 for Windows.
Questions included in the survey.
| 1. For inpatient from hematological stem cell transplantation or onco-hematology units, is the medical treatment always decided in common by the oncologist and intensivist? |
| 2. Do you think that treatment to these patients is always appropriate to its complexity and prognosis? |
| 3. What kind of monitoring is applied to critically ill pediatric hemato-oncology patient with hemodynamic instability and inotropic requirements? |
| 4. To treat respiratory failure or hypoxemia is non-invasive positive pressure ventilation (NIPPV) the first step of assistance? |
| 5. If NIPPV is applied do you use a protocol for its removal or substitution by another kind of assistance? |
| 6. Do you consider the initiation of conventional mechanical ventilation regardless of the underlying disease prognosis? |
| 7. Does your pediatric intensive care unit (PICU) have extracorporeal membrane oxygenation? |
| 8. Respiratory management if refractory hypoxemia or hypoventilation to conventional mechanical ventilation |
| 9. In case of oliguric acute kidney injury (creatinin≥2 over the limit for the patient age or twice the baseline) which of the following measures are never applied in your unit? |
| 10. Is convulsive status in critically ill pediatric hemato-oncology patient cause of admission to your PICU? |
| 11. If the patient died in PICU is he/she accompanied by an oncologist? |
| 12. Is there an oncologist on-call in your hospital? |
| 13. In case of critically ill pediatric hemato-oncology patient with a poor prognosis is the withdrawal of sustaining life support discussed? |
| 14. Is the decision of withdrawal sustaining life support agreed with the oncologist? |
| 15. Is the decision of withdrawal sustaining life support agreed with family? |
The survey was answered by 105 intensivists (32% of surveyed). In their opinion the treatment applied is always (18%) or frequently (78%) appropriate to CIPHOP complexity and prognosis (Table 2).
Medical care team.
| Do you think that treatment to these patients is always appropriate to its complexity and prognosis? | Never | Always | Rarely | Frequently |
| 0 (0%) | 20 (18%) | 3 (3%) | 82 (78%) | |
| Yes | No | Always if possible | ||
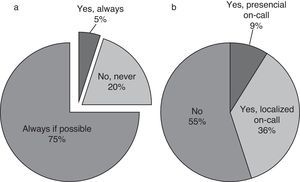
| For inpatient from HSCT or onco-hematology units the medical treatment is always decided in common by oncologist and intensivist? | 25 (24%) | 21 (19%) | 59 (56%) | |
HSCT: hematological stem cell transplantation. PICU: pediatric intensive care unit. CIPHOP: critically ill pediatric hemato-oncology patient. Answers (%).
The treatment and care giving decisions are made in common, if possible, by the oncologist and intensivist (56%; Table 2).
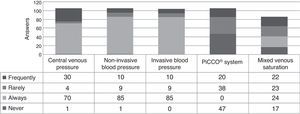
Hemodynamic monitoringNon-invasive blood pressure measurements are always used by 80% of respondents. It is associated with intra-arterial blood pressure measurements (always by 85/105) or central venous pressure (CVP; always by 70/105 and frequently by 30/105). The transpulmonar peripheric arterial thermodilution (PICCO®) is never or rarely applied. The mixed venous oxygen saturation is always used by 24/86 (Fig. 1).
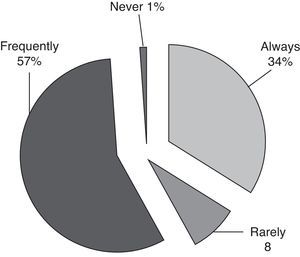
Respiratory managementIn case of respiratory failure or hypoxemia the non-invasive pressure support ventilation (NIPPV) is always (36/105) or frequently (60/105) used as first respiratory assistance (Fig. 2). Standardized protocols to replace or withdraw NIPPV are only used by 48% of the respondents (Table 3).
Respiratory management.
| Question | Yes | No |
|---|---|---|
| If NIPPV is applied do you use a protocol for its removal or substitution by another kind of assistance? | 46 (48%) | 50 (52%) |
| Do you consider the initiation of conventional mechanical ventilation regardless of the underlying disease prognosis? | 33 (31%) | 72 (69%) |
| Does your PICU has ECMO? | 30 (29%) | 75 (71%) |
PICU: pediatric intensive care unit. NIPPV: non-invasive positive pressure ventilation. ECMO: extracorporeal membrane oxygenation. Answers (%).
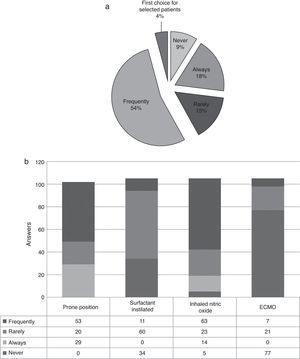
Before the initiation of conventional mechanical ventilation (MV) the patient prognosis is considered by 69% of respondents (Table 3, 72/105). In case of no improvement and/or hypoxemia the prone position (PP) is always used by 29/102. High oscillatory frequency ventilation (HOFV) is always applied if refractory hypoxemia despite MV or PP by 19/105 (Fig. 3). Pulmonary surfactant is used frequently by 10% of respondents (11/105). Inhaled nitric oxide is applied frequently (60%, 63/102) as therapy in case of refractory hipoxemia. The extracorporeal membrane oxygenation (ECMO) is used when necessary by 7/105 of intensivists (Fig. 3).
Renal managementIn cases of oliguric acute kidney injury (AKI) the continuous renal replacement therapy (CRRT) is used by 97% of respondents if no response to diuretic bolus or continuous infusion (Table 4).
Acute kidney injure and status epilepticus management.
| Renal management | |
|---|---|
| In case of oliguric AKI (creatinin≥2 over the limit for the patient age or twice the baseline) which of the following measures is never applied in your unit? | |
| Diuretics (bolus or perfusion) | 0 (0%) |
| Peritoneal dialysis | 12 (11%) |
| Hemodialysis | 28 (27%) |
| CRRT | 3 (3%) |
| All are applied | 62 (59%) |
| Status epilepticus | ||
|---|---|---|
| Is convulsive status in CIPHOP cause of admission to your PICU? | Yes | No |
| 102 (97%) | 3 (3%) | |
CIPHOP: critically ill pediatric hemato-oncology patient. CRRT: continuous renal replacement therapy. AKI: acute kidney injury. Answers (%).
Status epilepticus is considered as a cause of PICU incoming by 102/105 of the respondent intensivists (Table 4).
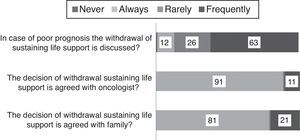
End-of-life decisions and careIn case of very bad prognosis the withdrawal of life-sustaining medical therapies is frequently discussed (63/102, 60%). This decision is taken in common by the oncologist and intensivist in 87% of cases (91/103); the caregivers participate always (81/103) or frequently (21/103) (Fig. 4). In case of death the oncologist is beside the patient always if possible (79%, Fig. 5).
DiscussionOur survey, the first done about this topic to European pediatric intensivists, was answered by one third of the Spanish intensivists registered in the Spanish Society of Pediatric Critical Care. Although there are no previous data about the global management of CIPHOP, almost all of the respondents considered their management adequate to the CIPHOP complexity and prognosis. Also, despite there not being a defined standard-of-care about the management of critically ill pediatric hemato-oncology patient, the respondents showed similar therapeutics choices to the different clinical situations examined. The procedures and therapeutic decisions appear to be similar to those taken in other critically ill children and end-of-life decisions are frequently decided in common by the intensivist and oncologist, in order to achieve adequate therapy for the children's requirements.
Answers discussionMedical care teamWe have seen that 19% of respondents have not integrated the collaboration with the oncology specialist in their clinical activity despite it being recognized as essential in CIPHOP treatment. It clearly helps to reduce inadequate decisions and supposes a corner stone in case of CIPHOP.7 The medical care team involved must be sensitive to individual requirements and, if it is possible, incorporate an interdisciplinary vision in this population.7
Hemodynamic monitoringIn our study the use of invasive hemodynamic measures in CIPHOP appears to be always done if indicated or necessary. As we have seen the measurement of arterial pressure, invasive or not, and CVP are globally included in CIPHOP management to the respondent.8 In case of the CVP maybe the requirement of a central venous line to apply the chemotherapeutics agents usually help to easily measure it.
The optimal mean arterial pressure (MAP) is uncertain and depends on patient characteristic and disease.9,10 Non-invasive blood pressure may be inaccurate and repeated mean pressure assessments are needed to optimize the hemodynamic treatment.8,11 In our study non-invasive monitorization is almost always the first measurement done to the CIPHOP later substituted by invasive methods if necessary.
The use of transpulmonar peripheric arterial thermodilution is considered by only 20% of respondents. Its use is feasible and seems safe in these children. The parameters derived from this monitorization could add clinically important information to asses the volume load in order to take adequate decisions about inotropic drugs or fluid therapy. Its low application could be associated with (1) low availability of this technology in the Spanish PICUs and (2) lack of experience and data to its interpretation.12,13
Finally in our study almost 40% of intensivists did not monitor mixed venous oxygen saturation usually. The maintenance of an optimal tissue oxygenation is an important task.14 There are many variables which are measured for this purpose.15 Nowadays the usefulness and interest to achieve an adequate monitoring of mixed venous oxygen saturation as an index of tissue oxygen delivery is clear11 and its application should be standardized in CIPHOP always if is indicated and possible.16
Respiratory managementThere are no prospective studies which confirm the NIPPV efficacy in immunocompromised children or CIPHOP. It seems that it could be applied without complications.17 The NIPPV is feasible and well tolerated17 and it could avoid intubation in almost half of the patients. It use is extended in the Spanish PICUs and has been applied by 96/105 of respondents as first respiratory assistance. The NIPPV should be initiated early, the clinical characteristics and severity scores between respondents and non respondents do not have clear differences.17 Despite the frequent use of NIPPV, 50% of participants did not apply a standardized protocol for its withdrawal or replacement.
In case of severe respiratory distress the conventional mechanical ventilation is indicated in the CIPHOP management.5 The acute respiratory distress syndrome (ARDS) is a common diagnosis among CIPHOP18,19 and usually evolves to a life threatening refractory hypoxemia.20 The ARDS is associated with significant risk of mortality despite the improvement over time in the general outcome.4 Almost 60% of children treated with hematopoietic stem cell transplantation (HSCT) develop ARDS related to the treatment itself or bacterial, viral or fungal infections. It is also a common phenomenon in non-HSCT oncology patients.
Several techniques are available to improve oxygenation in hypoxemic CIPHOP.21 The choice of MV strategy can significantly influence the course of lung injury, so the volutrauma and atelectrauma must be avoided by an adequate protective lung strategy.20,22 Avoid a dynamic hyperinflation applying low tidal volume by predicted body weight (6ml/kg) should be used as the only strategy that consistently improved outcome in ARDS.23,24 The positive end expiratory pressure (PEEP) should be used judiciously to maintain lung recruitment, prevent pulmonary atelectasis and facilitate synchrony in spontaneously breathing patients.25
Nowadays there is insufficient evidence to routinely recommend the use of HFVO,23 prone positioning,26 instilated surfactant or inhaled nitric oxide.20
The HOFV is applied by 81% of respondents, 4% of them choose HFVO as first therapeutic option in selected patients. The HFOV generally improves the ratio of partial pressure of oxygen to inspired fraction on oxygen (PO2/FiO2) and might improve survival.23,27
The role of prone position (PP) is not clear with controversial results in prospective studies.24,28 Several mechanisms can explain it benefits22 such an improving recruitment and more homogeneously distributing alveolar inflation.29 Prone position also influences the synthesis of some proinflammatory cytokines such IL-6 so it could have not only local effects. The prone position is a common therapy option of the intensivists in the study in case of hypoxemic respiratory failure with no response to MV.
The use of instilated surfactant is promising30 related to the extensive biochemical and biophysical changes of the pulmonary surfactant system in case of ARDS.21,31 This therapy is frequently applied by only 10% of those surveyed and it may be considered in children with direct lung injury.30 The optimum dosage and timing of administration are not clear.32 Tamburro et al. suggested it potentials benefits establishing the bases to future clinical trials.21 About inhaled nitric oxide its use is extensive by the respondents (73%) to achieve pulmonary vasodilatation and better oxygenation. There is no evidence that its use should be protocolized and it must be based on individual characteristics.
In relation to the use of ECMO there is a lack of data to clearly support its use in CIPHOP; it should be used in case of clinical features that suggest a reasonable indication. It has been recommended for severe ARDS because it avoids lung overdistension and the use of high oxygen concentrations. Its use in Spain is restricted to a short number of units. ECMO could be a therapy that may be appropriate for CIPHOP. It is necessary to have adequate entry criteria and treatment protocol in order to collect data from such experiences to advance the standard of care.
Renal managementThe acute kidney injury produces a high additional mortality and morbidity. An aggressive therapy is necessary mainly directed to achieve an adequate kidney perfusion and, as a consequence, the diuresis normalization. The use of diuretics by bolus or perfusion is usually the first therapy option.
In our survey the continuous renal replacement therapy (CRRT) is frequently applied by the respondents.33 In case of CIPHOP it could also be applied to treat fluid overload or as curative therapy for a great variety of disorders and multiorgan dysfunction (attenuate inflammation and improved oxygenation in mechanically ventilated children).33,34 The intensity is not yet well defined but it is known that high-dose CRRT in AKI does not improve patient survival or recovery function. Related to this, recently Rajasekaran et al. described in a retrospective study that CRRT is not associated with long-term survival in pediatric with HSCT.34
Status epilepticusAlmost all the respondents consider as a cause of PICU income the presence of status epilepticus. In case of CIPHOP the seizures could be derived from the oncological disease, the radiotherapy or chemotherapy treatment and the infections.35 In our survey there were no questions about the pharmacological treatment. Actually some novel pharmacological agents such as levetiracetam are recommended.36,37
End-of-life decisionsIt is known that the majority of deaths in PICU require discussions or decisions about when or how to limit support by withdrawal of life-sustaining medical therapy.5,38 This situation is frequently common in case of CIPHOP. The work in a children's hospital is focused on treatments aimed toward cure. Death is often seen as a failure, and it may not be discussed or acknowledged as a possibility until very late in a child's stay in hospital. About the care of dying child or about end-of-life decisions in children there is no right model of care moreover that pain and symptom management must be intensified to achieve an adequate quality of life. It appears clear that open discussions about treatment discontinuation are necessary in order to generate better interventions.7,39 Understandable information to the parents about child health status influences their ability to participate in end of life decisions.40 Both things, collaborative work and family participation, are well considered and applied by the intensivist consulted. Those who refer an absence of the oncology in case of death are probably related to not having an on-call oncologist in their hospital.
There are several limitations to our study. It was conceived as an observational transversal study so the results are influenced by the number of respondents. Besides, based on the answers and in the survey characteristics, it was only possible to make a descriptive analysis. Also our survey was not standardized, because there are no similar previous studies or papers. Related to the study designs there are multiple confusion factors that were impossible to discard (critical care unit complexity or number of CIPHOP admissions per year and cause of them for example). The anonymous design, with no questions about the PICU characteristic of each participant, influenced the answers and the results should be related with a personal perception of how these patients should be treated instead of how they are really treated. Future observational and prospective studies should be performed to describe PICU treatment in pediatric oncological patients to acquire knowledge about what is really done and, if necessary, set new or different therapies.
ConclusionsDespite there not being a standard-of-care, Spanish intensivists showed an elevated concordance about the therapeutic decisions taken in case of critically ill pediatric hemato-oncology patient. Related to this, the use of NIPPV as first respiratory assistance is extended. The results of this survey do not establish new treatment criteria or protocols, but it could be used as a base to know the global management of CIPHOP in order to develop future studies and consensus.
Conflict of interestThe authors declare no conflict of interest to report.
We want to thank all the critical care physicians for their availability and kindness, and the members of the working Group on the critically ill or unstable hematooncological patient (paciente oncohematológico grave o inestable [OncoCIPed]) of the Sociedad Española de Cuidados Intensivos Pediátricos (Spanish Society of Pediatric Critical Care).
Please cite this article as: García-Salido A, Nieto-Moro M, Iglesias-Bouzas MI, González-Vicent M, Serrano-González A, Casado-Flores J. Paciente crónico oncohematológico, ¿hacemos lo que deberíamos hacer? An Pediatr (Barc). 2016;85:61–69.