Since the COVID-19 pandemic was declared in March 2020, we have learned a lot about the SARS-CoV-2 coronavirus, and its role in pediatric pathology.
Children are infected in a rate quite similar to adults, although in most cases they suffer mild or asymptomatic symptoms. Around 1% of those infected require hospitalization, less than 0.02% require intensive care, and mortality is very low and generally in children with comorbidities. The most common clinical diagnoses are upper or lower respiratory infections, gastrointestinal infection and, more seriously, multisystemic inflammatory syndrome (MIS-C). Most episodes do not require treatment, except for MIS-C. Remdesivir has been widely used as a compassionate treatment and its role has yet to be defined.
The newborn can become infected, although vertical transmission is very low (<1%) and it has been shown that the baby can safely cohabit with its mother and be breastfed. In general, neonatal infections have been mild.
Primary care has supported a very important part of the management of the pandemic in pediatrics. There has been numerous collateral damage derived from the difficulty of access to care and the isolation suffered by children. The mental health of the pediatric population has been seriously affected. Although it has been shown that schooling has not led to an increase in infections, but rather the opposite. It is essential to continue maintaining the security measures that make schools a safe place, so necessary not only for children's education, but for their health in general.
Desde que en marzo de 2020 se declarara la pandemia COVID-19 hemos aprendido muchas cosas del coronavirus SARS-CoV-2, y de su papel en la enfermedad pediátrica.
Los niños se infectan en un porcentaje bastante similar a los adultos, si bien en la mayoría de las ocasiones sufren cuadros leves o asintomáticos. Alrededor de un 1% de infectados precisan hospitalización, menos de un 0,02% precisan cuidados intensivos, y la mortalidad es muy baja y generalmente en niños con comorbilidades. Los cuadros clínicos más habituales son infecciones respiratorias de vías altas o bajas, cuadros gastrointestinales y con mayor gravedad el síndrome inflamatorio multisistémico (MIS-C). La mayoría de los episodios no precisan tratamiento, salvo el MIS-C. El remdesivir se ha empleado generalmente como tratamiento compasivo y aún está por definir su papel.
El recién nacido puede infectarse, si bien la transmisión vertical es muy baja (< 1%), y se ha demostrado que el bebé puede cohabitar de manera segura con su madre y recibir lactancia materna. En general las infecciones neonatales han sido leves.
La atención primaria ha soportado una parte muy importante del manejo de la pandemia en pediatría. Se han producido numerosos daños colaterales derivados de la dificultad de acceso a la asistencia y del aislamiento que han sufrido los niños. La salud mental de la población pediátrica se ha visto seriamente afectada. A pesar de que se ha demostrado que la escolarización no ha supuesto un incremento de los contagios, sino más bien todo lo contrario. Es fundamental seguir manteniendo las medidas de seguridad que permitan hacer de las escuelas un lugar seguro, tan necesario no solo para la educación infantil, sino para su salud en general.
On December 31, 2019, the Wuhan Municipal Health Commission (Hubei province, China) reported the detection of cases of severe pneumonia with a common history of visiting a seafood and wild animal market. On January 7, 2020, the Chinese authorities informed that the causative agent had been identified, a new virus in the Coronaviridae family that was eventually named SARS-CoV-2. The genome sequence was divulgated on January 12, 2020.1 On January 30, the World Health Organization declared the outbreak a public health emergency of international concern,2 and on March 30 a global pandemic.
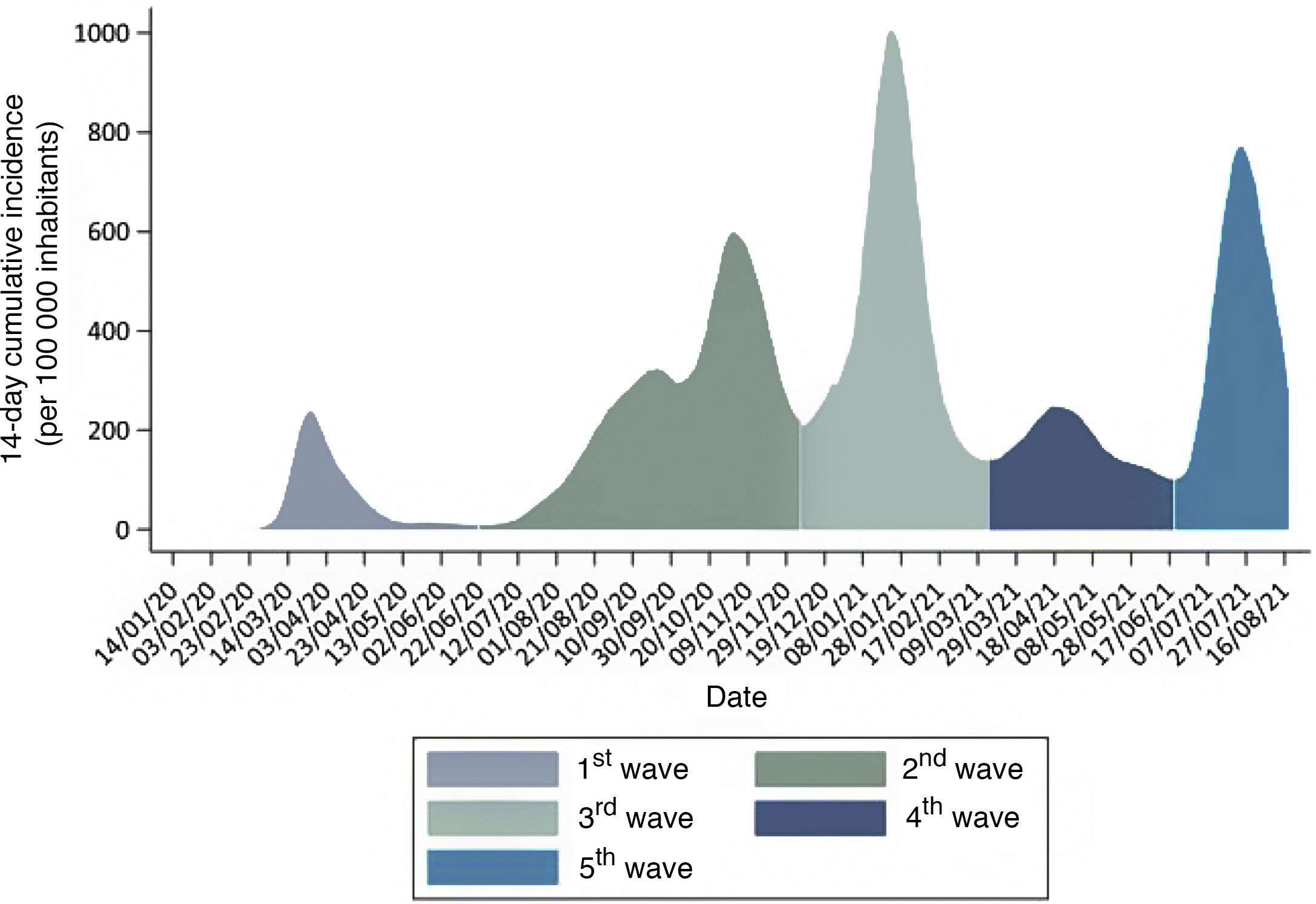
In Spain, from that point through August 18, 2021, the COVID-19 pandemic has unfolded in 5 waves or periods3 (Fig. 1):
- -
First wave of the pandemic: from the outset until June 21, 2020, when the state of alert was called off in Spain.
- -
Second wave: from June 22 to December 6, 2020, the inflection point of the 14-day cumulative incidence between waves.
- -
Third wave: from December 7, 2020 to March 14, 2021, the inflection point of the 14-day cumulative incidence of coronavirus disease 2019 (COVID-19) between the third and fourth waves.
- -
Fourth wave: from March 15, 2021 through June 19, the inflection point of the 14-day cumulative incidence of COVID-19 between the fourth and fifth waves.
- -
Fifth wave: from June 20, 2021 to present.
The impact on the paediatric population has changed through time in terms of both its characteristics and the perception of knowledge of the disease, due, among other things, to the varying availability of diagnostic tests. At the beginning of the pandemic, the polymerase chain reaction (PCR) test was only performed in children with severe disease managed at the hospital level. Thus, in the first wave of the pandemic in Spain, approximately 1400 cases of infection by SARS-CoV-2 were detected in children under 14 years, which amounted to 1% of the total cases diagnosed. Among these children, 26% required inpatient care, and 54 children required admission to the paediatric intensive care unit (PICU) and 3 died (0.2% of the total). In the second wave, when diagnostic tests became widely available, with performance of antigen and antibody tests and contact tracing, the number of paediatric cases increased sharply until they amounted to 12% of the total diagnosed, with a hospitalization rate in children of 0.8%, a PICU admission rate of 0.02% and a mortality of less than 0.01%.3 The mortality is estimated at 0.21 per 100 000 children aged 0–9 years and 0.34 per 100 000 children aged 10–19 years.4 This temporal trend has been similar in Spain and the rest of developed countries.5
The fourth report of the National Study on the Serological Epidemiology of SARS-COV-2 Infection in Spain (ENE-COVID),6 of December 2020, suggests that 400 000 children in Spain had seroconverted at that point. Taking into account that at least 25% of children do not develop detectable levels of antibodies, the actual number that got infected is probably higher.
In the Barcelona area, the data showed that the rate of infection was similar in children and adults sharing a household, although, as it is known, the course in the former is much milder or asymptomatic.7 The presentation of detected cases has changed between the first and second waves, with a decreasing proportion of patients with severe respiratory disease (pneumonia) and an increasing proportion of febrile illness and mild infection.8 Although there are fewer data for the third and fourth waves, it appears that severity and the frequency of hospitalization continue to decline, and it is unclear whether these changes are due to the emergence of new variants or to our improved knowledge of the disease.
Clinical characteristics and underlying diseases of infected individualsThe clinical spectrum of COVID-19 in children is broad, with symptoms including, in order of decreasing frequency, fever and low-grade fever, cough, rhinorrhoea, vomiting, abdominal pain, diarrhoea, fatigue, headache, sore throat, breathing difficulty, myalgia and, more rarely, rash, conjunctivitis, enanthema, wheezing, chest pain, arthralgia and changes in taste and smell. The following broader syndromes or phenotypes have been described: mild disease (upper respiratory tract infection indistinguishable from other aetiologies, flu-like illness), fever without source, lung disease (usually viral pneumonia and, much less frequently, bronchitis, asthma attacks or bronchiolitis), gastrointestinal disease (abdominal pain, vomiting, diarrhoea) and multisystem inflammatory syndrome in children (MIS-C).9 The latter is a severe form of illness following infection by SARS-CoV-2 that is due to immune dysregulation and that usually requires intensive care. Thrombosis, on the other hand, has not been a frequent complication in children.10 In the subset of children that require hospital admission, the risk factors for severity include elevation of inflammatory markers, target organ damage (elevated creatinine, low oxygen saturation), leucocytosis, neutrophilia, lymphopaenia, anaemia and thrombocytopenia. Online models are available to predict which patients are at high risk of severe COVID-199 (https://rserver.h12o.es/pediatria/EPICOAPP/, user name: user, password: 0000).
The paediatric study of infections by novel coronavirus SARS-CoV-2 (EPICO-AEP), which encompasses 10% of the 800 public and private hospitals in Spain, had recruited approximately 650 hospitalised patients as of March 2021. In Spain, 19% of children with COVID-19 managed at the hospital level had some form of comorbidity, a proportion that rose to 28% in the case of patients admitted to hospital and 60% in patients admitted to the PICU. Most of the patients that died (90%) had a severe underlying disease. There is evidence that underlying cardiac disease, liver disease and asthma is associated with an increased risk of admission to the PICU. Interestingly, patients with asthma were admitted to the PICU due to pneumonia, as opposed to asthma exacerbations. In addition, neurologic comorbidities, obesity and immunosuppressive therapy have been associated with an increased risk of MIS-C. In contrast, cancer, rheumatic disease and diabetes have not been associated to an increased risk of severe COVID-19 in children in Spain.9 In other regions, type 1 diabetes has been associated with severe disease.11
In children, acute disease can be diagnosed by means of the rapid antigen test or the PCR test in an appropriate sample. Nasopharyngeal (NP) swabs are the most frequently used samples, but recent data from the EPICO-AEP suggest that the PCR test in buccal swab saliva specimens processed and analysed the same way as NP swabs have a similar diagnostic yield that is also greater compared to the rapid antigen test. In children, the rapid antigen test is less accurate than in adults (sensitivity of 60%–72% compared to the PCR test).12
Nevertheless, it is important to take into account that a positive PCR test does not always correspond to the acute phase of infection. The mean time to PCR-negative conversion in children is 17 days, independently of age, severity, phenotype or immunosuppression.13
Treatment of infection by SARS-CoV-2The decision to initiate treatment in children with COVID-19 is complicated, as most children have mild symptoms that resolve with symptomatic treatment and there is little evidence on the use of different treatments in the paediatric population.14
In children who develop moderate to severe disease within a few days of infection, when the viral load is still high, administration of antivirals could curtail replication.14,15 Remdesivir is the only antiviral to date that has achieved a reduction in the length of stay in clinical trials, especially when administered early.14,15 It has been approved for use in children over 12 years. In younger children, it is used on a compassionate basis.14,16 Previous case series report few adverse events in children with severe COVID-19 and resolution in most cases.14,15
Dexamethasone could be beneficial to some patients with severe disease.16 This drug reduces morbidity and mortality in seriously ill patients receiving mechanical ventilation, while in patients with milder presentations it does not improve outcomes compared to placebo.16 Other immunomodulatory drugs, such as tocilizumab, baricitinib or anakinra, have been used in adults, but the current evidence of their use in children with COVID-19 is scarce.14
In hospitalised adults, administration of convalescent plasma has been found to reduce morbidity and mortality, especially if given early on and if it contains high antibody titres of antibodies.14,17 In patients with COVID-19 with risk factors that are not hospitalised, early outpatient treatment with neutralising antibodies (bamlanivimab and casirivimab-imdevimab [REGN-COV2]) has proven effective in reducing the progression to severe disease.18 However, the use of convalescent plasma and monoclonal antibodies in children is controversial, the efficacy data in this population is scarce, they may cause infusion-related reactions and it is difficult to determine which patients are at risk of developing severe disease and could benefit from their early administration.14,18 Although they should not be used routinely, their use could be individualised in select patients.14,18
As for the management of MIS-C, the most frequent approaches have been steroid therapy, in boluses or at lower doses, and intravenous immunoglobulin, on account of the similarities to Kawasaki disease.19 Some authors have used them in combination, although recently published data obtained in 614 children with MIS-C failed to evince the superiority of any of these 3 options compared to the rest.19 Therefore, any of these approaches could be used while we await stronger evidence.
In short, in children with moderate symptoms, especially if they have an underlying disease that puts them at risk of severe COVID-19, the use of remdesivir in the early days of infection could be beneficial. Furthermore, dexamethasone is recommended in children with prolonged disease, especially those requiring respiratory support. In children with MIS-C, initial treatment may consist of immunoglobulins or steroids as monotherapy or a combination of both.
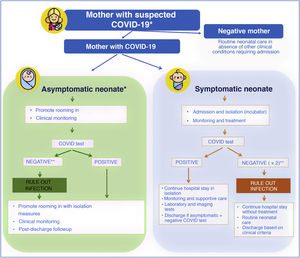
Impact of COVID-19 on neonatal clinical practiceNeonates can also become infected by SARS-CoV-2, but, in addition to the mechanisms of transmission at play in all age groups, in this particular population there is a specific mechanism which is vertical transmission from a mother infected by SARS-CoV-2 to the foetus or neonate, which may occur in utero (congenital infection), during childbirth (perinatal infection) or postnatally through the breast milk.20
When we analysed the impact of COVID-19 on the mother-child dyad, we found that pregnant women with COVID-19 are at greater risk of severe disease (admission to intensive care unit), general and obstetric complications and death.21,22 Thus, there is evidence of a significant increase in the frequency of preterm birth in infected pregnant women, in most cases associated with severe maternal disease (medically indicated early-term delivery to improve maternal health), which results in poorer neonatal outcomes in children of mothers with COVID-19.23
When it comes to vertical transmission, cases of congenital infection have been rare, with a very low incidence of perinatal infection (<1%), and there has been no proven case of transmission through breast milk. In fact, most cases reported in infants were of postnatal transmission through the usual mechanisms and from the close contacts of the infant (mother, other relatives etc). In addition, the presentation in infants tends to be asymptomatic or mild (symptoms of upper respiratory tract infection and/or fever and/or diarrhoea) or does not differ in any way from the presentation of other neonatal diseases (preterm infants).24,25
The low impact on infants could be related to maternal passive immunity, as there is evidence of the transplacental transfer of IgG antibodies against SARS-CoV-2 in infants of infected or vaccinated mothers, and neutralising antibodies against SARS-CoV-2 (IgG and IgA) have been detected in human milk.26,27
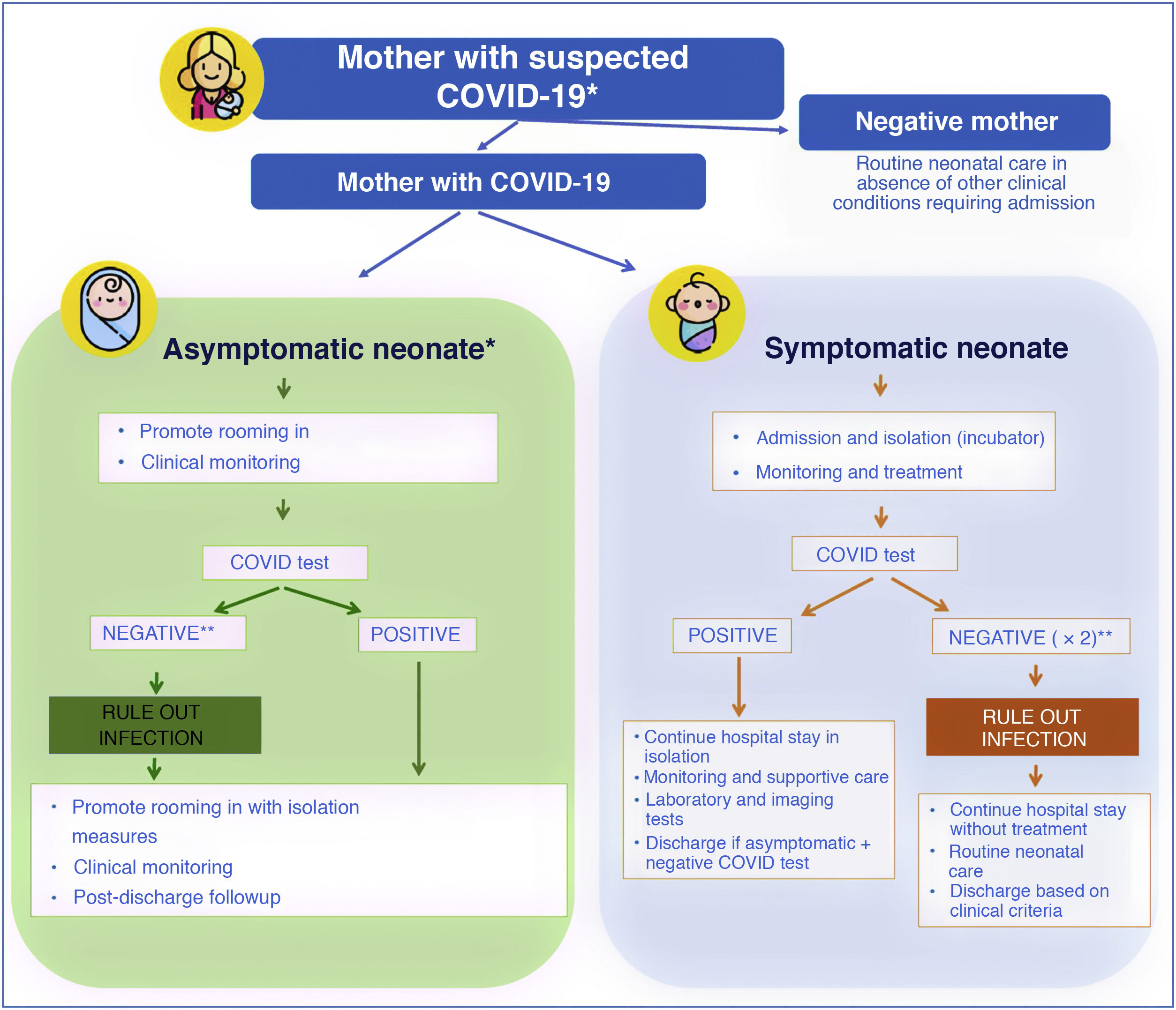
At the beginning of the pandemic, the Sociedad Española de Neonatología (Spanish Society of Neonatology, SENeo) published guidelines for the management of neonates in the context of SARS-CoV-2, and especially for infants born to mothers with COVID-19. Our stance from the outset, despite the scarcity of the evidence, was to avoid the separation of the newborn infant and the mother (as long as the clinical condition of both allowed it, and irrespective of SARS-CoV-2 test results in the infant) and to maintain breastfeeding (Fig. 2). At the same time, to obtain evidence of our own, we instituted the COVID-19-SENeo register to collect data on infected pregnant women and their infants and on cases of postnatal infection by SARS-CoV-2 (nosocomial or acquired in the community). Having analysed 15 months’ worth of data collected in more than 3700 mother-infant dyads, we did not find any confirmed case of congenital infection, and found an incidence of perinatal infection of less than 1%. When it came to cases of postnatal infection, with a total of 138 recorded cases included in the analysis, we found that 20% were asymptomatic, 72 were mild and 8% required admission to the intensive care unit.
Management of neonates born to mothers with suspected SARS-CoV-2 infection.
* In case of mothers undergoing evaluation or that tested positive, if the mother is oligosymptomatic or asymptomatic, rooming in of mother and infant is recommended with contact and droplet isolation measures (hand hygiene, face mask and crib 2 m apart from mother’s bed) and promotion of breastfeeding.
** In symptomatic infants born to mothers with confirmed infection or strong suspicion of infection based on clinical/epidemiological factors, ruling out infection requires 2 negative viral PCR tests (at birth and at 24−48 h). In asymptomatic infants, 1 or 2 PCR tests will be done depending on test availability.
Lastly, this pandemic has also had a collateral impact on infants derived from the limits placed on parental presence in neonatal units (restrictions on family-centred care, etc) and the stress experienced by health care workers.
COVID-19 and primary care (Table 1)Disease managementFrom the early days of the pandemic, primary care paediatricians (PCPs) identified possible COVID-19 cases in patients and their contacts, recommended isolation measures, followed up patients and watched for warning signs and symptoms (MIS-C and severe respiratory disease) and assessed the need for referral. Once PCR and antigen tests became available, it was possible for PCPs to confirm the diagnosis, which made it possible to limit losses of schooling or work time to confirmed cases and to the minimum necessary duration.28
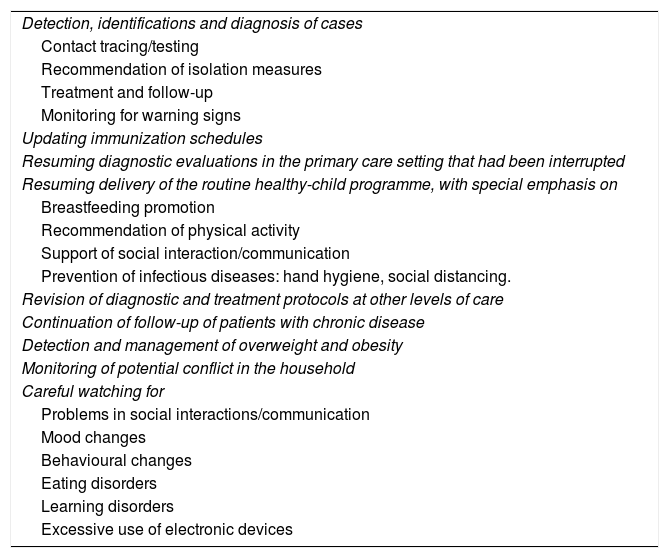
Role of primary care paediatricians in the context of COVID-19.
| Detection, identifications and diagnosis of cases |
| Contact tracing/testing |
| Recommendation of isolation measures |
| Treatment and follow-up |
| Monitoring for warning signs |
| Updating immunization schedules |
| Resuming diagnostic evaluations in the primary care setting that had been interrupted |
| Resuming delivery of the routine healthy-child programme, with special emphasis on |
| Breastfeeding promotion |
| Recommendation of physical activity |
| Support of social interaction/communication |
| Prevention of infectious diseases: hand hygiene, social distancing. |
| Revision of diagnostic and treatment protocols at other levels of care |
| Continuation of follow-up of patients with chronic disease |
| Detection and management of overweight and obesity |
| Monitoring of potential conflict in the household |
| Careful watching for |
| Problems in social interactions/communication |
| Mood changes |
| Behavioural changes |
| Eating disorders |
| Learning disorders |
| Excessive use of electronic devices |
It was assumed that the start of the school year and the expected increase in seasonal infectious diseases would complicate care delivery, but there has been a substantial decrease in the incidence of non-COVID infectious diseases, including the absence of the flu season,29 probably associated with the lockdown and hygiene (frequent handwashing and use of masks) and social distancing measures.
Collateral impactThe decreased delivery of in-person care at the PC level and in hospitals, the prioritization of the management of COVID-19 patients, the closure of schools and recreational activities and rehabilitation services and home confinement have had an impact on health. The most important are:
- -
Important decrease in vaccine coverage in the early months of the pandemic.30 We do not know whether interrupted vaccination schedules have been generally caught up with by now.
- -
Delays in testing, diagnosis and treatment initiation. Adequate coordination between levels of care is necessary to remedy this situation.
- -
Children who require early intervention services and stimulation, physical therapy, occupational therapy, psychotherapy, speech therapy, etc, access to which is significantly delayed under normal circumstances, have experienced interruptions in care, and there has been a significant delay in the assessment of children referred to determine the need of these services. This constitutes a significant loss of opportunity to improve their situation.
- -
In addition to the general lockdown, there have been many other local confinement measures, with closure of parks and recreational activities. This, combined with the anxiety of families, has severely limited opportunities for social interaction outside the household. Depending on the developmental stage at which these measures took place, they may have affected the development of social skills in children. Close follow-up of these children is required to differentiate delays in skill acquisition from actual disorders.
- -
An increase in obesity, probably related to what was noted in the previous point and the increase in sedentary habits.
- -
As has been the case at other levels of care, we have detected a significant increase in the number of visits related to mental health complaints, some involving excessive use of electronic devices.31–33 We must take into account that many families have lost members under conditions of isolation and loneliness, that others have become impoverished and that confinement measures may have been triggers in violent or dysfunctional households. The resources devoted to managing mental health issues are clearly insufficient.
- -
The pandemic has impoverished many families. Due to the saturation of social services and the closure of school meal services, the basic needs of children are not being met.
During the COVID-19 pandemic, there have been situations that threatened the physical and mental health of children, the impact of which will be felt in the short and the long term. We must remain watchful at the primary care level to detect any possible disorders.34
Transmission of SARS-CoV-2 by children and implications for in-person schoolingUntil the explosive fifth wave in summer of 2021, Spanish children and adolescent seemed to have been comparatively spared by the COVID-19 pandemic, despite being as susceptible to infection as adults.7 Although at the beginning of the pandemic it was hypothesised that the youngest in the population could play a role as superspreaders, as is the case when it comes to other respiratory viruses, such as influenza, it soon became clear that children had a more limited role in the transmission of SARS-CoV-2 to other individuals. The few studies on the subject available to date suggest that children are rarely the source of superspreading events, that large outbreaks are infrequent in children under 12 years, and that children seemed to be less infectious compared to adults,35,36 with the ability to infect others increasing with age, reaching the transmission potential of adults in late adolescence. A school-based study conducted in Catalonia allowed estimation of these differences in transmission potential in terms of the reproduction number (R*), an epidemiological parameter that stands for the average number of secondary infections produced by one case, and found R values that were 30% (for young children) and 60% (for older children) the reference R value in adults.37 This measure was calculated in children attending schools that had implemented strict preventive measures, so the resulting values must be interpreted in the context of a transmission containment strategy.
The reopening of schools for in-person learning in September 2020 was perceived as a risky choice (that has been extensively contested) by large segments of the population in the context of the incipient second wave that was ravaging the country. However, a careful analysis of data on infections in the school-age population indicates that schools have not been hotbeds of transmission (if anything, they appear to have done the opposite) and that the measures implemented in the facilities have dramatically contained outbreaks in this setting and reduced transmission in the classroom. Even at times when the transmission rate was alarmingly high in Spain, reaching an incidence of nearly 1000 cases per 100 000 inhabitants, schools were able to continue their activity without major changes, with fewer than 5% of the established bubbles needing to be quarantined due to detection of a positive case and approximately 75% of detected infections not giving rise to any secondary cases (unpublished data). These data are reassuring when it comes to the return to school in September 2021, but the high transmission rate recorded in children in recent months and the emergence of variants that are much more infectious suggest the need to maintain the strict measures applied in the previous school year and of including children aged 12 or more years in the national SARS-CoV-2 vaccination campaign to make vaccination a requisite to attend schools, thus making the return to the classroom, if at all possible, even safer.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Calvo C, Tagarro A, Méndez Echevarría A, Fernández Colomer B, Albañil Ballesteros MR, Bassat Q, et al. Pandemia COVID-19. ¿Qué hemos aprendido en este tiempo? An Pediatr (Barc). 2021;95:382.