The management of congenital lung malformations (CLMs) is controversial because most of them are asymptomatic. The aim of our study was to analyze the association of preoperative symptoms with surgical outcomes in patients with CLMs treated by means of minimally invasive surgery (MIS).
Patients and methodsRetrospective study of patients with CLM treated with MIS in our hospital between 2003 and 2023. We recorded data on the following variables: demographic characteristics, size, type and location of the lesion, mediastinal shift, clinical symptoms, surgical time, conversion to open surgery, duration of chest drainage (days), length of stay and postoperative complications.
ResultsThe sample included 116 patients, 98 (84.4%) asymptomatic and 18 (15.5%) with clinically significant symptom: infectious in 10 (55.5%) and respiratory distress in eight. The most frequent histopathological type was congenital pulmonary airway malformation (35.5%), followed by intralobar sequestration (27.3%), and the lower lobes were most commonly involved (78.4%). Larger lesion size on the CT scan was significantly associated with the development of symptoms (P = .027) and with an increased frequency of conversion to open surgery. However, the incidence of postoperative complications was not greater in the symptomatic group.
ConclusionsPostoperative complications were not significantly more frequent in the symptomatic group of patients. Larger lesion size on imaging was associated with a significantly increased probability of developing symptoms, so these patients may benefit from early surgical resection.
El tratamiento de las malformaciones pulmonares congénitas (MPC) es controvertido ya que la mayoría de ellas cursan de forma asintomática. El objetivo principal de este estudio fue analizar el impacto de la presencia de sintomatología previa en el curso intra y postoperatorio en los pacientes intervenidos de MPC mediante cirugía mínimamente invasiva (CMI).
Material y métodosEstudio retrospectivo de pacientes con MPC tratados con CMI en nuestro centro desde 2003 hasta 2023. Se analizaron las siguientes variables: datos demográficos, tipo y tamaño de la lesión, localización, desplazamiento mediastínico, sintomatología, tiempo quirúrgico, conversión a cirugía abierta, días con drenaje torácico, estancia hospitalaria, y complicaciones postoperatorias.
ResultadosSe incluyeron 116 pacientes, 98 (84.4%) asintomáticos y 18 (15.5%) sintomáticos: 10 (55.5%) de tipo infeccioso y 8 dificultad respiratoria. El tipo histológico más común fue el correspondiente a malformación congénita de la vía aérea pulmonar (35.5%) seguido de secuestro intralobar (27.3%), y los lóbulos inferiores fueron los más afectados (78.4%). El tamaño de la lesión en la TC fue significativamente más grande en el grupo de pacientes que desarrollaron sintomatología (p = 0.027) y la tasa de conversión a cirugía abierta fue también mayor en este grupo. No se identificaron más complicaciones postoperatorias en el grupo de pacientes con síntomas previos.
ConclusionesNo se demostró un aumento significativo de las complicaciones postoperatorias en el grupo de pacientes con clínica previa. Las lesiones más voluminosas detectadas en pruebas de imagen tienen un riesgo significativamente mayor de desarrollar sintomatología y son candidatas a una cirugía precoz.
Congenital lung malformations (CLMs) form a heterogeneous group of anomalies among which so-called congenital pulmonary airway malformations (CPAMs) stand out on account of their frequency, with an estimated incidence of 1–5 per 10 000 births.1–6 The management of these lesions is controversial; since most of them are asymptomatic, there are authors that recommend a watchful waiting approach.5,7–11 However, elective surgical resection continues to be the approach preferred by most groups on account of the risk of malignant transformation (especially in some types of CPAM) and the potential infectious or respiratory complications, which develop in up to 30% of cases. In the case of symptomatic patients, there is widespread consensus that the appropriate approach is early surgical intervention with resection of the lesion.12–22
The primary objective of the study was to assess the potential impact of symptoms present before surgery in intraoperative and postoperative outcomes in patients who underwent correction of a CLM by minimally invasive surgery (MIS). We also aimed to identify factors that could play a role in the development of symptoms in this subset of patients. Our working hypothesis was that patients with CLMs that present symptoms before surgery may experience more complications during or after surgery compared to asymptomatic patients, so that early detection of certain lesion characteristics could help identify patients who are more likely to develop symptoms over time and could benefit from early surgical intervention.
Material and methodsStudy designWe conducted a retrospective study of patients with a prenatal diagnosis of CLM managed in a tertiary care referral hospital between October 1, 2003 and October 3 l, 2023 through the review of the records of a prospective database. The data were collected and managed through the Research Electronic Data Capture (REDCap) platform of our hospital. REDCap is a secure web-based application designed to support data capture for research studies that offers: (1) a user-friendly interface to collect validated data; (2) a built-in logging and audit trail to track any manipulation of the data; (3) automated export features that allow seamless download of data to commonly used statistical software packages, and (4) modules to import data from external sources.
Data collectionWe included every patient with a diagnosis of CLM who underwent surgical intervention in our hospital between October 1, 2003 and October 31, 2023 (20-year period). The cohort was divided in two groups based on whether or not the patient had clinical manifestations associated with the CLM, collecting data on the following variables: demographic characteristics, type and size of lesion, mediastinal shift, symptoms, procedure duration, conversion to open surgery, days of chest drainage, length of stay and postoperative complications. We classified symptoms as infectious in the case of symptoms secondary to pneumonia ipsilateral to the CLM. We defined respiratory symptoms as those requiring some form of respiratory support (invasive or noninvasive) before surgery. Patients who received a prenatal diagnosis of CLM and remained asymptomatic after birth underwent a chest computed tomography (CT) scan with intravenous contrast between three and eight months post birth. Patients with features suggestive of malignancy in the prenatal ultrasound (CPAM type 4 or pleuropulmonary blastoma based on the Stocker classification),23 relevant respiratory manifestations or pneumothorax in the neonatal period, the CT scan was performed early in the first month of life. All patients underwent MIS performed by the same surgical group. The thoracoscopic lobectomy technique has been widely reported in the literature and follows the basic principles of the open chest surgery approach.24 At the end of the procedure, a chest drain was placed and a follow-up chest X-ray performed in the intensive care unit.
Statistical analysisWe have expressed quantitative variables in terms of median and range and categorical variables in terms of absolute frequency and percentage distributions. In the comparative analysis, we used the Kruskal-Wallis test to assess differences in continuous variables between groups. In the case of categorical variables, we used the χ2 test or, when the frequency of the event required, the Fisher exact test. The analysis was conducted with the Stata software package, version 16 (Stata Corp. 2019; College Station, TX, USA). In all tests, we considered p values of less than 0.05 statistically significant.
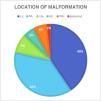
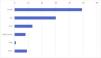
ResultsThe sample included 116 patients, of who 98 (84.4%) were asymptomatic and 18 (15.5%) had clinical manifestations associated with the pulmonary malformation before surgery. In the symptomatic group, ten patients (55.5%) had symptoms associated with infection and eight had respiratory distress (2 managed with mechanical ventilation, 3 with noninvasive ventilation [CPAP] and 3 with supplemental oxygen). In our study, symptoms were most frequent in female versus male patients (P = .040) and malformations were most frequently located in the inferior lobes (78.4%) (Fig. 1). The most frequent histological type was CPAM (35.5%, followed by intralobar sequestration (27.3%) and extralobar sequestration (11.8%) (Fig. 2). In the group of symptomatic patients, the histological distribution was as follows: CPAM (9 cases), intralobar sequestration,4 extralobar sequestration,2 congenital lobar overinflation2 and pleuropulmonary blastoma1 (Fig. 3). Considering the variables analyzed in both groups of patients (symptomatic vs asymptomatic), we found a significantly greater lesion size in the group of patients who developed symptoms (P = .027) (Fig. 4). Although the difference was not statistically significant, we also found a higher frequency of mediastinal shift in the prenatal ultrasound in symptomatic patients. As regards the potential impact of the presence of symptoms before surgery in intraoperative and operative outcomes, the rate of conversion to open surgery was higher in the symptomatic group, without a significant impact on length of stay (Table 1). On the other hand, the frequency of postoperative complications was not greater in the symptomatic group (Table 1). The complications documented in the cohort were: bleeding, persistent air leak, severe subcutaneous emphysema, prolonged fever and sepsis.
Analyzed variables in each group of the cohort.
| Variables | Asymptomatic | Symptomatic | P |
|---|---|---|---|
| Sex, female/male | 45/53 | 13/5 | .040 |
| Age at surgery, median (months) | 12 | 14.6 | .869 |
| Weight, median (kg) | 9.45 | 9.25 | .645 |
| Lesion size on CT, median (cc) | 33.5 | 58.5 | .027 |
| Location: | |||
| LID | 40 | 4 | .135 |
| LM | 4 | 2 | .215 |
| LSD | 5 | 2 | .325 |
| LII | 40 | 7 | .661 |
| LSI | 7 | 3 | .185 |
| Abdominal | 2 | 0 | .541 |
| Mediastinal shift | 25 | 7 | .053 |
| Operative time, median (minutes) | 150 | 167 | .360 |
| Chest drainage, median (days) | 4 | 3 | .735 |
| Length of stay, median (days) | 5 | 7 | .077 |
| Postoperative complications | 26 | 4 | .684 |
Statistically significant values are presented in italics.
Although there is widespread consensus regarding the need of surgical resection in patients with CLM who develop infectious or respiratory symptoms, there is disagreement regarding the indication of surgery in asymptomatic patients.7–11 This lack of consensus is even greater when it comes to the optimal timing of surgical resection. Although the recent development of MIS techniques has allowed safe performance of lung excision procedures in increasingly young patients, the best time to perform the surgery has yet to be determined. Different authors have compared surgical outcomes in asymptomatic patients of various ages, always under one year, and found no differences in postoperative outcomes or the development of complications.4,13 Research has also been conducted to assess the potential impact of the presence of preoperative symptoms on postoperative outcomes. Aspirot et al.25 found a significant increase in postoperative complications and length of stay in patients who were symptomatic before surgery. However, other authors have not found a significant association in this regard.21,26 Our study did not find a significant increase in postoperative complications in patients who were symptomatic before surgery, but conversion to open surgery due to technical complications was more frequent in this group, although it was not associated with an increase in operative time. Patients with a history of pneumonia involving the lobe where the lesion is located may have adhesions and hilar lymphadenopathy at the time of surgery. This makes the resection procedure longer and more complicated, with a higher risk of bleeding, which may motivate conversion to open surgery, as was the case in two patients in our cohort.27,28 Given that the incidence of pulmonary infection in patients with CLMs can range from 10% to 30% in the first year of life, several authors recommend surgical resection of the lesion in infancy to prevent potential infectious diseases that could compromise the expected outcomes of MIS.5,13,18,29,30 Although the data from our cohort did not allow detection of a significant association, it is reasonable to surmise that, had the sample been larger, early intervention would probably had a positive impact on the conversion rate. Another argument in support of early surgical intervention is the potential for malignancy in some CLMs, and more specifically in CPAMs.31 It is still unclear whether cystic lesions undergo malignant transformation or the malformation is malignant from the outset, but, either way, the incidence is very low.21 In our cohort, only one patient had histological features of malignancy compatible with BPP, corresponding to a proportion of 0.86% that was consistent with the findings published by other research groups.13,32
We also analyzed the potential association of certain prenatal findings or malformation characteristics with the development of postnatal symptoms. The detection of hydrops and/or mediastinal shift in the prenatal ultrasound have been described as predictors of a poor prognosis in the literature, in addition to large lesion size.33–35 In our study, the size of the lesion measured in the postnatal CT scan was significantly greater in the symptomatic group, but the difference in the frequency of mediastinal shift in the prenatal ultrasound, which usually results from the mass effect of a large lesion, was not statistically significant, probably due to the small sample size. Although the information obtained through imaging is very useful and increasingly precise, the correlation between the findings of the fetal ultrasound and the postnatal CT scan is still weak.36 We ought to highlight the relatively low incidence of hybrid malformations (CPAM + sequestration) in our cohort. This is probably due to the fact that lesions initially considered hybrid in the diagnostic CT scan were later reclassified as intralobar sequestration based on the histopathological findings. Advances in the knowledge and accuracy of histological techniques in recent years may account for this difference.
There are certain limitations to our study. First, its retrospective design and relatively small sample size, which can limit the performance of more complex statistical analyses, as was the case of the rate of conversion to open surgery. In addition, we included two patient subtypes in the symptomatic group: those with manifestations of respiratory distress and those with infectious symptoms. Although these types of clinical manifestations may end up having a somewhat different impact on intraoperative and postoperative outcomes, most studies in the literature combine them into a single category, as symptomatic patients are a group that differs clearly from asymptomatic patients.26,37
In conclusion, although the results obtained in our study did not evince a significant increase in postoperative complications in the group of patients who were symptomatic before surgery, certain outcomes, such as the rate of conversion to open surgery, may suggest that early intervention would be beneficial in this group. In addition, a larger lesion size in the prenatal ultrasound confirmed in the postnatal thoracic CT scan is associated with a significantly increased risk of developing symptoms, so these patients are also candidates for surgical resection before age one year.
Ethical considerationsThe study was approved by the Research Ethics Committee of the hospital (code 21/393) and we obtained informed consent of the parents or legal guardians of all patients for the surgery and the acquisition and use of photographs and video recordings.