The treatment of complicated pleural infection (CPI) is controversial. Clinical guidelines recommend drainage, but with the lowest grade of evidence. Recent reports have observed good outcomes with antibiotics alone. We retrospectively compared the outcomes in two consecutive cohorts treated with different policies: the first treated according to pleural fluid characteristics (2005–2009, interventional-prone, group 1) and the second according to clinical assessment (2010–2013, conservative-prone, group 2).
MethodsThe clinical records of all children treated for CPI in our hospital between 2005 and 2013 were thoroughly reviewed. Primary outcomes were the proportion of children drained and the length of hospital stay (LHS).
ResultsOne hundred and nine patients (64 group 1 and 45 group 2) were analyzed. A chest tube was placed in 83% of patients in group 1 and 47% in group 2 (P<0.001). The mean LHS was 11.4 days for patients in group 1 and 12.3 for patients in group 2 (P=0.45); no differences were observed in other outcomes.
ConclusionOur results add to few recent observations reporting good outcomes in many children treated with antibiotics alone and challenge the need to drain most children with CPI. Clinical trials are now needed to identify when a drainage procedure would be useful.
El tratamiento del derrame pleural paraneumónico complicado (DPC) es controvertido. Las principales guías recomiendan el drenaje, pero con el menor nivel de evidencia. En trabajos recientes se han observado buenos resultados solo con antibióticos. Hemos comparado retrospectivamente nuestros resultados en dos cohortes consecutivas de pacientes tratados con distinto criterio: en el grupo 1 (2005–2009, actitud intervencionista) el drenaje se decidía en función de las características del líquido pleural, de acuerdo con las principales guías; en el grupo 2 (2010–2013, actitud conservadora) el drenaje se decidía en función de la evolución clínica del paciente.
MétodosSe revisaron las historias clínicas de los pacientes tratados por DPC entre 2005 y 2013. Las principales variables analizadas fueron la proporción de pacientes drenados y la duración de la estancia hospitalaria.
ResultadosSe analizaron 109 pacientes (64 grupo 1 y 45 grupo 2). Se colocó un tubo de drenaje en el 83% de los pacientes del grupo 1 y en el 47% de los del grupo 2 (P<0,001). La duración media de la estancia hospitalaria fue de 11,4 días en el grupo 1 y 12,3 días en el grupo 2 (P=0,45). No se observaron otras diferencias destacables.
ConclusiónNuestros resultados coinciden con los de otros estudios recientes que han observado una buena evolución en niños tratados solo con antibióticos y cuestionan la necesidad del drenaje en muchos de los niños que padecen un DPC. Son necesarios ensayos clínicos para identificar las circunstancias en las que se puede obtener un beneficio del drenaje en el DPC.
Pleural effusion and empyema are occasional complications of pneumonia in children that have been reported to have increased in recent years.1–4 Small effusions do not require invasive procedures for diagnosis and treatment, and do well with antibiotics alone. The treatment of large, organized and purulent effusions (fibrinopurulent stage) remains controversial and different approaches have been reported, from conservative antibiotic treatment to chest tube insertion or other surgical procedures, mainly video-assisted thoracoscopic surgery. Most guidelines consider that complicated pleural infection (CPI) should not be managed with antibiotics alone and recommend drainage,5–8 but some institutions have observed good outcomes without requiring a chest tube or surgery for many of the affected children, suggesting a benefit from a more individual approach.9,10
Our institution is the reference center for a population of almost 250,000 children under 15 years old when they require intensive care or pediatric surgery. Many children diagnosed with CPI are transferred from other hospitals for evaluation and treatment. In 2010, as a result of our experience and a recent report,9 we moved from our previous adherence to authorized guidelines, resulting in draining most cases of CPI, to a more conservative and individualized management. We report our experience in the last 9 years, and compare patient characteristics, treatments and outcomes of children attending our hospital from 2005 to 2009, when a chest tube was usually inserted, depending on the characteristics of the effusion, with those who attended from 2010 to 2013, who were managed according to holistic clinical criteria, mainly the severity of appearance and respiratory difficulties based on physical examination.
Patients and methodsSelection of patientsInformation on patients younger than 15 years old admitted between 2005 and 2013 inclusively, with a discharge diagnosis of pleural effusion or empyema, was retrieved from our hospital electronic database. The clinical records of these patients were retrospectively reviewed to select those with pleural effusion associated with a diagnosis of community-acquired pneumonia. Patients with an effusion associated with tuberculosis or other diseases were excluded. Parapneumonic effusions were considered to be CPI if the pleural space was bigger than 1cm as measured in plain chest roentgenogram and loculations or debris were observed by pleural ultrasound. Only patients with CPI were selected for analysis.
Hospital managementPatients were admitted from the emergency department of our hospital or transferred from other hospitals. Two groups of patients are compared: those admitted between 2005 and 2009 (group 1) were managed according to the British Thoracic Society guidelines for the management of pleural infection in children,5 and those admitted between 2010 and 2013 (group 2) were treated according to the clinical assessment of the attending pediatricians. The clinical findings driving a decision to drain the empyema in patients of group 2 included increased effort of breathing and severe or persistent septic condition.9,10 Persistent fever was not considered a reason to drain the effusion, because it has been reported to be long-standing irrespective of whether they are drained or not.9 Chest tube insertion was usually performed by an interventional radiologist assisted by an anesthetist. The child was maintained under deep sedation and a small-bore pigtail catheter was inserted, with ultrasound guidance, using the Seldinger technique. Less frequently, a chest tube was inserted by a pediatric surgeon, more often for children admitted to the intensive care unit (ICU). Thoracentesis and computed tomography scan were usually not performed. All other decisions (chest tube removal, length of antibiotic treatment, discharge from hospital, etc.) were taken according to the best clinical judgment of the attending physicians.
Data collectionThe clinical files of the patients selected were thoroughly reviewed and data were extracted for each patient, including sex, age, length of the hospital stay (LHS), comorbidities, signs and symptoms, duration of fever, blood and pleural fluid laboratory and bacteriological results, antibiotics administered, length of antibiotic treatment, oxygen therapy, admission to ICU, drain tube insertion, surgical interventions and complications. Pneumococcal vaccination status could not be analyzed due to inconsistent registration.
OutcomesThe primary outcome measures for comparison between groups 1 and 2 were (1) the proportion of children with a chest tube inserted in our hospital and (2) the LHS. Secondary outcome measures for analysis were: treatments employed, length of antibiotic treatment and duration of fever. Length of primary fever was computed as the sum of number of days of fever before admission and the time to defervescence, considered as the time (in days) elapsed between admission and the first day with a temperature under 38°C for 24h. Fever reappeared in some patients after defervescence. In such cases, the total number of days with fever during admission and the total number of days from admission to complete disappearance of fever were calculated.
StatisticsData were transferred to SPSS (v17.0 SPSS, Chicago, IL, USA). Descriptive analysis included frequencies and percentages for categorical variables, means, standard deviation (SD) and range for numerical variables with a normal distribution, and medians, interquartile range (IQR, 25th and 75th percentiles) and range for numerical variables with a non-normal distribution. Categorical variables were compared using the Fisher exact test or the chi-squared test for trend. Continuous variables were compared by means of t-test (with normal distribution) or Mann–Whitney U test (with non-normal distribution). Reported P values were two-tailed, and the level of significance was set at 0.05.
EthicsThe study was approved by the Clinical Research Ethics Committee of our hospital.
ResultsSelection of patientsTwo hundred children were discharged from our hospital between 2005 and 2013 with a diagnosis of pleural effusion. Twenty-four of them were not of infective origin and five were diagnosed with tuberculosis. The remaining 171 children had a parapneumonic pleural effusion and were selected for detailed review of their clinical records. Sixty-two had small simple effusions and were not considered to need a drainage procedure. The other 109 had large, complicated effusions, deemed to need drainage according to the main guidelines. These 109 patients were considered to have CPI and were the subject of our study. Sixty-four children were admitted in 2005–2009 (12.8/year) and 45 in 2010–2013 (11.3/year).
Patients’ characteristicsThe main characteristics of patients on admission are depicted in Table 1. A history of asthma or recurrent wheezing was present in 10% of patients, a frequency very close to that of the general pediatric population in our country.11 One patient had cerebral palsy, two had epilepsy and another two had Down's syndrome. Two children had lesions of current chickenpox. Most of the patients were previously healthy or had other problems unrelated to the development of pneumonia or a pleural effusion.
Characteristics of children with complicated pleural infection on admission.
| 2005–2009 (n=64) | 2010–2013 (n=45) | P | |
|---|---|---|---|
| Male sex, n (%) | 38 (59) | 20 (44) | 0.17 |
| Age, years, median (IQR) | 3.2 (2.1–5.4) | 3.4 (1.8–4.8) | 0.46 |
| Children aged 1–5 years old, n (%) | 50 (78) | 32 (71) | 0.50 |
| Transferred from another hospital, n (%) | 46 (72) | 33 (73) | 1.00 |
| History of asthma/wheezing, n (%) | 7 (11) | 4 (9) | 1.00 |
| Fever before admission, days, mean (SD) | 5.2 (2.8) | 4.7 (2.7) | 0.08 |
| Cough, n (%) | 53 (83) | 39 (87) | 0.79 |
| Respiratory distress, n (%) | 39 (61) | 33 (73) | 0.22 |
| Chest pain, n (%) | 21 (33) | 14 (31) | 1.00 |
| Leukocytes×109/L (blood), mean (SD) | 20.1 (8.9) | 20.4 (8.5) | 0.88 |
| Neutrophils×109/L (blood), mean (SD) | 14.5 (8.1) | 15.8 (7.6) | 0.39 |
| C-reactive protein, mg/dL, mean (SD) | 21.4 (11.1) | 23.7 (12.8) | 0.31 |
| Left side affected, n (%) | 32 (50) | 31 (69) | 0.08 |
All patients had fever at some stage of the disease. Before admission, two patients had low-grade fever (less than 38°C) for only one day. The other 107 had fever (38°C or over) ranging from 1 to 10 days. The frequency of cough, respiratory distress and chest pain is shown in Table 1. Other common signs and symptoms were (n): vomiting (28), abdominal pain (23), diarrhea (13), headache (6) and febrile seizure (2).
In patients drained, pleural fluid median values (IQR) were: protein 4g/L (4–5), glucose 10mg/dL (2–31), leukocyte count 3.4×109/L (0.6–22.8). Bacterial cultures were positive in 30 patients (28%): Streptococcus pneumoniae was recovered from 17 patients (16%: 9 blood, 8 pleural fluid), Staphylococcus epidermidis from 4 (3 blood, 1 pleural fluid), Streptococcus pyogenes from 3 (1 blood and 3 pleural fluid), Staphylococcus aureus from 2 (1 blood, 1 pleural fluid) and other bacteria from 4 patients.
General treatmentThe treatments are compared in Table 2. Patients usually received more than one antibiotic, including oral antibiotics before and/or after hospitalization. Antibiotics were administered for a period of 7–34 days, but the mean was 2 days longer in the second group; the intravenous route was used for 0–26 days. Fifty-four patients needed oxygen for a median of 4 days (IQR 3–7 days, range 1–15 days). Thirteen patients were admitted to the pediatric ICU for a mean of 8.3 days (SD 6.9 days, range 1–23 days), but only two required assisted ventilation.
Treatment of children with complicated pleural infection.
| 2005–2009 (n=64) | 2010–2013 (n=45) | P | |
|---|---|---|---|
| Cefotaxime, n (%) | 51 (80) | 37 (82) | 0.81 |
| Amoxicillin–clavulanate, n (%) | 49 (77) | 36 (80) | 0.82 |
| Vancomycin, n (%) | 32 (50) | 16 (36) | 0.17 |
| Macrolide, n (%) | 12 (19) | 6 (13) | 0.60 |
| Total duration of antibiotics,a days, mean (SD) | 17.6 (5.0) | 19.9 (4.8) | 0.02 |
| Duration of intravenous antibiotics,a days, mean (SD) | 11.4 (4.7) | 12.0 (4.2) | 0.50 |
| Oxygen administration, n (%) | 27 (42) | 27 (60) | 0.08 |
| Stay in intensive care, n (%) | 8 (13) | 5 (11) | 1.00 |
| Chest tube, n (%) | 53 (83) | 21 (47) | <0.001 |
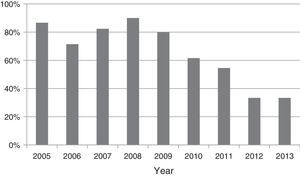
A chest tube was placed in our hospital in 74 patients (68%): 83% of patients in group 1 and in 47% in group 2, and this was the most noticeable difference in their treatment (Table 2). Urokinase was administered in 85% of those drained (91% in group 1 and 71% in group 2, P=0.07). There was no significant trend in the percentage of patients drained between 2005 and 2009 (P=0.91), but a progressive reduction of chest tube placement was found between 2009 and 2013 after the change in treatment policy for empyema (P=0.006) (Fig. 1). Thoracoscopy was performed in just one patient, due to treatment failure after 9 days with a chest tube. Two patients admitted to our center in 2009 had been drained before being transferred from their hospital. Exclusion of these two patients made no difference in the comparisons of treatments and outcomes.
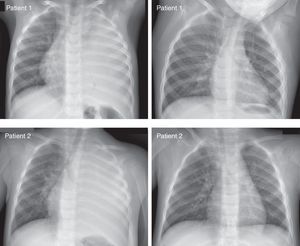
OutcomesThe main outcomes are depicted in Table 3. A 1-year-old girl transferred to our center in 2005 had a chest tube inserted, but was transferred the next day to another specialized center due to a pericardial effusion, where she was treated and recovered. This patient was excluded from the analysis of duration of antibiotics and outcomes. LHS (mean 11.8 days, SD 6.1, range 3–32 days) did not differ significantly between the two groups, even when the two patients drained in another hospital were excluded. Primary fever lasted 11.6 days (SD 5.0 days, range 1–25 days) and the time to defervescence after admission ranged from 0 to 21 days. Fever reappeared after defervescence during hospital stay in 22 patients after an apyretic period of 1–8 days, and the new fever lasted 1–12 days. There were no differences between groups in the total number of days with fever during admission (mean 7.7 days) and the total number of days from admission to complete disappearance of fever (mean 8.4 days). Only one patient presented pneumothorax before chest tube insertion. The mean LHS was longer in children with pneumothorax (18.0 vs. 10.6 days, P<0.001), even when only drained patients were analyzed (18.0 vs. 12.1 days, P<0.001). Although long-term outcomes were not systematically registered, all patients recovered and no permanent damage was observed on follow-up. Fig. 2 shows the initial and last chest X-ray of two representative patients with very large undrained CPI.
Main outcomes of children hospitalized for complicated pleural infection.
| 2005–2009 (n=63a) | 2010–2013 (n=45) | P | |
|---|---|---|---|
| Length of hospital stay, days, mean (SD) | 11.4 (5.8) | 12.3 (6.7) | 0.45 |
| Time to defervescence since admission, days, mean (SD) | 6.8 (4.9) | 6.9 (4.2) | 0.86 |
| Time to defervescence since admission longer than 12 days, n (%) | 6 (10) | 3 (7) | 0.73 |
| Reappearance of fever after defervescence, n (%) | 11 (17) | 11 (24) | 0.47 |
| Pneumothorax, n (%) | 9 (14) | 8 (18) | 0.60 |
Patients who were drained were similar to those who were not drained in their main characteristics on admission, but a right effusion was more commonly drained than a left one (80% vs. 59%, P=0.02). There were no differences in the antibiotics employed and the total length of antibiotic treatment, but drained patients had a longer duration of intravenous antibiotics (12.5 vs. 9.8 days, P=0.004), and more frequently required oxygen (59% vs. 29%, P=0.004) and ICU admission (18% vs. 0%, P=0.009). LHS was longer in drained patients (13.5 vs. 8.1 days, P<0.001), even if patients with pneumothorax were excluded (11.1 vs. 8.1 days, P<0.001).
DiscussionMuch controversy surrounds the diagnosis and management of parapneumonic pleural effusion and empyema. Pleural infection is a continuum classically divided into stages. In line with BTS guidelines and other authors, we have diagnosed CPI (empyema included) when the fibropurulent stage was evidenced by the presence of a large (>1cm) effusion with echographic signs of organization.5,8 Since biochemical analysis has not been shown to be of any value in the practical management of children with infective pleural effusions, aspiration of pleural fluid was not normally performed solely for that purpose.5,12 When pleural fluid was available, results of biochemical and bacteriological analysis clearly showed that our patients were affected by complicated effusion and empyema.
Unlike other studies searching for the best procedure for draining, the current study suggests that conservative management with antibiotics alone is a suitable way to treat many children with CPI. This study adds to a few recent similar observations,9,10 and widens the controversy about the best treatment for CPI, largely due to the lack of trials to answer this question. A recent systematic review found only four randomized trials and concluded that, in children who require pleural drainage, fibrinolysis could be as effective as video-assisted thoracic surgery and more effective than placebo to reduce LHS.13 Another systematic review of studies comparing video-assisted thoracoscopic surgery versus chest drain with fibrinolytics arrived at similar conclusions.14 In line with these results and most guidelines, most of our drained patients were treated with fibrinolytics. However, there are no trials comparing conservative versus invasive management of CPI. Antibiotic treatment alone is generally not recommended in current guidelines for CPI, but this recommendation has the lowest grade of evidence.5 CPI is feared given the risk of death or permanent damage, possibly coming from past or adult experiences.15–17 However, the mortality in healthy children is remarkably low, and the long-term functional and radiological outcome is reported to be excellent.18–24 Accordingly, the management of CPI should be directed toward decreasing short-term morbidity, patient discomfort, LHS, and the cost of treatment.
Epaud et al. evaluated the outcome of 65 patients with infective pleural effusion. In the first 15 months a chest tube was inserted according to the characteristics of pleural effusion (classical group). During the following 15 months, patients were drained according to clinical criteria (conservative group). Significantly fewer chest-tube insertions were performed in the conservative group (25% vs. 52%, P=0.03). The duration of temperature over 39°C was shorter in the conservative vs. classical group (10 vs. 14 days, P=0.01), without significant differences in LHS (16 days vs. 20, P=0.16).9 More recently, Carter et al. published their 12-year experience of conservative treatment of pleural empyema in children. Patients who were clinically stable were started on IV antibiotics alone. If the patient's clinical condition did not improve within 48–72h, a drainage procedure was performed. Ninety-five of 182 patients (52%) were treated with antibiotics alone while 48% underwent drainage procedures, mainly those who needed ICU care or had large pleural effusions. Patients treated with antibiotics alone had a shorter LHS, fewer hospital days with fever, and briefer courses of IV antibiotics than those undergoing drainage procedures. There were six complications from drainage procedures: one patient had a liver laceration and five developed pneumothoraces. Their results suggest that some children with moderate or large parapneumonic effusions can be effectively managed with antibiotics alone: even 23% of patients with mediastinal shift were successfully managed this way.10 Similar results have been observed in adult patients.25 In a recent retrospective review of almost 15,000 children admitted with parapneumonic pleural effusion and empyema, significant variation in primary interventions between 40 USA children's hospitals was observed.26 Treatment with antibiotics alone was the primary intervention in 52% of patients, ranging between 30 and 75% depending on the hospital. Interestingly, LHS and costs were lower in patients treated with antibiotics alone than in patients receiving invasive procedures, despite having a comparable severity of disease as shown by the need for mechanical ventilation and ICU. These findings underscore the importance of establishing which patients truly require drainage.
Altogether, this and other reports clearly point out that the natural history of CPI in children treated with antibiotics and supportive measures usually lead to complete resolution. Fever is a bad indicator for improvement, because it usually persists for many days before subsiding, independently of the placement of the chest tube.9,27 It is not fever remission, but a very slow recovery (or just no worsening in the first few days) that points to a favorable outcome and precedes fever remission and healing.10 On the other hand, invasive treatments are risky, painful, expensive and dependent on professional skills and sanitary resources.28,29 Pneumothorax was observed in a number of patients after the chest tube insertion and resulted in a significantly longer LHS. We do not know to what extent pneumothorax was related to the disease or to an iatrogenic complication of chest tube insertion, because both possibilities have been reported.9,30
Our work has the limitations of most other observational studies in this field, from a single institution, with a limited sample size. Nevertheless, patients in the two study periods were almost identical on admission and the only difference in the management was the policy regarding draining the effusion, resulting in a lower rate of chest tube insertion and a slightly longer total (mainly oral) antibiotic treatment, but similar outcomes. Patients who were drained in the second group were probably sicker or did not improve with antibiotics. We did not attempt to point out which patients should or should not be drained, or the complex reasons for that decision. Relying on clinical judgment for intervention has been adopted even in clinical trials in other respiratory diseases.31 Instead, we have verified that some patients with CPI may avoid an invasive procedure and have a similar good progress. It is worth mentioning that a change in the policy regarding draining CPI from radiological to clinical criteria did not lead to a rapid but rather a progressive change in the number of patients not drained. Given the subjective nature of the interpretation of clinical findings, the clinicians became more confident about conservative treatment as clinical experience with this new policy showed good outcomes.
In conclusion, many children with CPI could be treated with antibiotics, avoiding unpleasant, costly and risky invasive procedures. Randomized trials are finally needed to elucidate when a drainage procedure may actually benefit a child affected by CPI.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Moral L, Loeda C, Gómez F, Pena MÁ, Martínez M, Cerdán JM, et al. Derrame paraneumónico complicado: análisis de dos cohortes consecutivas tratadas con distinto criterio. An Pediatr (Barc). 2016;84:46–53.
Preliminary results of this work have been presented to the XXXIV Reunión de la Sociedad Española de Neumología Pediátrica, San Sebastián, 2012.