Coinfections of influenza and other respiratory viruses (ORVs) are frequent in the epidemic season. The aim of this study was to examine the demographic and virological variables associated with coinfections by influenza and ORVs.
Materials and methodsWe analysed respiratory samples of patients with laboratory-confirmed influenza using molecular diagnostic methods obtained in 8 consecutive influenza seasons (2011–2012 to 2018–2019). We analysed data focusing on different variables: age, sex, type of patient (hospitalized/sentinel) and detected type/subtype of influenza.
ResultsCoinfections of influenza and ORVs were detected in 17.8% of influenza-positive samples. The probability of detecting coinfection was significantly higher in young children (0–4 years; OR: 2.7; 95% CI: 2.2–3.4), children (5–14 years; OR: 1.6; 95% CI: 1.2–2.1) and patients infected with the A(H3N2) subtype (OR: 1.4; 95% CI: 1.14–1.79). Also, we found a significantly higher frequency of coinfections involving influenza and 2 or more other respiratory viruses in young children (0–4 years; OR: 0.5; 95% CI: 0.32–0.8), adults (40–64 years; OR: 0.5; 95% CI: 0.3–0.9) and women (OR: 0.7; 95% CI: 0.5–0.9).
DiscussionThese results show that coinfections of influenza and ORVs are more frequent in young children and children, and in cases involving the A(H3N2) influenza subtype. Our findings can be useful to guide the use of multiplex diagnostic methods in laboratories with limited resources.
Las coinfecciones por gripe y otros virus respiratorios (OVR) durante las epidemias gripales son frecuentes. El objetivo de este estudio es examinar las variables demográficas y virológicas relacionadas con las coinfecciones entre la gripe y OVR.
Materiales y métodosEn este estudio se analizaron muestras respiratorias de 8 epidemias gripales consecutivas (desde la temporada 2011–2012 hasta la temporada 2018–2019), en las que se había detectado un resultado positivo de gripe mediante test en laboratorio. Analizamos los datos objetivándolos frente a diferentes variables: edad, sexo, tipo de paciente (hospitalizado/centinela) y tipo/subtipo de gripe detectada.
ResultadosLas coinfecciones entre gripe y OVR se detectaron en el 17,8% de los casos positivos de gripe. En los niños de entre 0-4 años (OR: 2,7; IC 95%: 2,2–3,4), los niños de entre 5-14 años (OR: 1,6; IC 95%: 1,2–2,1) y los pacientes infectados por el subtipo A(H3N2) (OR: 1,4; IC 95%: 1,14–1,79), se detectó una probabilidad significativamente mayor de detectar estas coinfecciones. Además, observamos que las coinfecciones entre gripe y 2 o más OVR fueron llamativamente más frecuentes en niños de 0–4 años (OR: 0,5; IC 95%: 0,32–0,8), en adultos de entre 40–64 años (OR: 0,5; IC 95%: 0,3–0,9) y en mujeres (OR: 0,7; IC 95%: 0,5–0,9).
DiscusiónEstos resultados muestran que las coinfecciones entre gripe y OVR son más frecuentes en niños de 0–4 años y de 5–14 años, y en los casos en los que el subtipo A(H3N2) está implicado. Estos datos pueden ser útiles para enfocar el diagnóstico mediante métodos multiplex en aquellos laboratorios que posean pocos recursos económicos y humanos.
Influenza is a respiratory disease that causes epidemics during the winter months. However, during these epidemics, respiratory viruses other than influenza are also circulating, such as human coronaviruses (hCoVs), rhinoviruses, and respiratory syncytial virus (RSV).1 This cocirculation makes the detection of coinfections of influenza and other respiratory viruses (ORVs) frequent.2,3
Currently, the microbiological diagnosis of respiratory viruses is mainly performed using molecular techniques. Among those methods, multiplex polymerase chain reaction (PCR) allows detection of several microorganisms in the same sample.4 In fact, a variety of molecular techniques for syndromic diagnosis of respiratory infections have proliferated in recent years. However, not all of laboratories have the same capacity, facilities, and economic and human resources to set up these multiplex techniques, and some of them have to use other, simpler methods that can only detect influenza or at most one more respiratory virus in the same reaction, frequently RSV.
The importance of detecting coinfections of influenza and ORVs has not been established clearly. In children, some studies have shown that coinfection is not associated with the severity of disease,5–7 although other studies have found an association with increased severity, especially if RSV or rhinovirus are involved.8–13 In adults, there seems to be greater consensus that coinfections are relevant, especially in patients who suffer from special clinical conditions, such as immunosuppression.14–17
The aim of our study was to describe the demographic and virological factors associated with the probability of detecting coinfection of influenza and ORVs though multiplex PCR methods, and to determine which patients would benefit most from this multiplex detection method.
Materials and methodsWe conducted a retrospective observational study that included 3623 respiratory samples (nasal wash in children aged less than 2 years and nasopharyngeal swab in older children) from patients with laboratory-confirmed influenza detected by multiplex RT-PCR obtained over 8 consecutive influenza seasons (2011–2012 to 2018–2019). The respiratory samples were collected from patients admitted to different hospitals of Castilla y León (Spain) and patients managed in sentinel outpatient sites of the Castilla y León Influenza Sentinel Network. Viral identification tests were performed at the National Influenza Centre-Microbiology Unit of the Hospital Clínico Universitario de Valladolid, Spain. For each patient, we collected demographic data (age, sex and type of patient [inpatient/sentinel]) and virological data (type/subtype of influenza [H1, H3 and B], ORV in coinfection) from the laboratory request forms. We defined coinfection as detection in a respiratory sample of 1 or more ORV in addition to influenza. The study adhered to the principles of the Declaration of Helsinki, and it was exempt from informed consent on account of the clinical and diagnostic use of the samples.
MethodsRespiratory viruses were detected with the Luminex 200 system and the RVP XTAG® Fast v2 reagents from 2011 to 2015, and with the MAGPIX detection system and the NxTAG-RPP reagents from 2016 to 2019 (Luminex, Austin, TX, USA). This multiplex PCR technique uses a battery of primers for amplification of the genetic material, and subsequently hybridization is performed with microspheres labelled for detection in the MAGPIX system.18 These reagents detect the following respiratory viruses: adenovirus, bocavirus, hCoV-HKU1, hCoV-NL63, hCoV-OC43, hCoV-229E, metapneumovirus, rhinovirus/enterovirus, influenza A, A(H1N1)pdm09 and A(H3N2) subtypes, influenza B, parainfluenza virus 1, 2, 3 and 4, and RSV A and B. The Luminex diagnostic system is cannot differentiate between enterovirus and rhinovirus, so it presents them as a single diagnostic target.
Statistical analysisWe performed the statistical analysis based on the different demographic and virological parameters documented in laboratory request forms, such as age, sex, type of patient and the detected type/subtype of influenza detected. We divided the sample into 5 age groups commonly used in studies on influenza and ORVs: young children (0–4 years), children (5–14 years), young adults (15–39 years), adults (40–64 years) and elderly (≥65 years). We used the Mann–Whitney U test to compare the median age in patients with and without coinfection. We analysed the association between the independent variables under study (demographic and virological) and the presence of coinfection by fitting a multivariable logistic regression model, expressing the results as the odds ratio (exp(B)). The statistical analysis was performed with the software SPSS version 20, and we set an α level of 0.05.
ResultsSample characteristics and influenza infectionsDuring the follow-up period, a total of 17 708 respiratory samples were analysed, of which 3623 (20.5%) were positive for influenza. Out of those, 1808 (49.9%) tested positive for influenza A(H3N2), 971 (26.8%) for influenza A(H1N1)pdm09, and 844 (23.3%) for type B influenza. The median age of the sample was 50.0 years (interquartile range [IQR], 66). The proportion of male patients was 51.2%, and 74.0% of the patients were hospitalized. We found the highest number of influenza positive samples in the group aged 65 years or older (n = 1311; 36.2%), and the lowest in the group aged 15-39 years (n = 382; 10.5%) (Table 1). The A(H3N2) subtype was the one detected most frequently in every age group, with a higher proportion of this subtype compared to other influenza viruses in the group aged 65 years or older (n = 807; 61.6%), and a similar proportion compared to influenza B in the group aged 5–14 years (A(H3N2): n = 223, 42.4%; B: n = 206, 39.1%).
Median age (IQR), number and percentage of influenza-positive samples, and type/subtype detected by age group under study.
| Age group | Median age (IQR) | Influenza-positive samples | % | Detected influenza virus | ||
|---|---|---|---|---|---|---|
| A(H3N2) | A(H1N1)pdm09 | B | ||||
| n (%) | n (%) | n (%) | ||||
| 0–4 years | 2.0 (2.0) | 647 | 17.9% | 260 (40.1%) | 222 (34.3%) | 165 (25.6%) |
| 5–14 years | 8.0 (5.0) | 526 | 14.5% | 223 (42.4%) | 97 (18.5%) | 206 (39.1%) |
| 15–39 years | 32.0 (12.0) | 382 | 10.5% | 183 (47.9%) | 118 (30.9%) | 81 (21.2%) |
| 40–64 years | 52.0 (11.0) | 757 | 20.9% | 335 (44.3%) | 266 (35.1%) | 156 (20.6%) |
| ≥65 years | 79.0 (14.0) | 1311 | 36.2% | 807 (61.6%) | 268 (20.4%) | 236 (18.0%) |
IQR, interquartile range.
In the period under study, there were 645 identified coinfections of influenza and ORVs (17.8% of the total cases of influenza virus detection). Out of these, 490 (76.0%) involved only 1 other respiratory virus and 155 (24.0%) 2 or more ORVs in addition to influenza. The virus detected most frequently in coinfection with any type of influenza virus was rhinovirus/enterovirus (n = 160; 24.8%), followed by RSV (n = 155; 24.0%), bocavirus (n = 123; 19.1%), and hCoV (n = 118; 18.3%).
The analysis by age showed that patients without coinfection had a significantly higher median age (41.0; IQR, 58) compared to patients with coinfection (23.0; IQR, 68) (Mann–Whitney U; P < .05). Coinfections of influenza and ORVs were more frequent in young children (age 0–4 years, n = 199; 30.8%) and children (age 5–14 years, n = 109; 20.7%) compared to other age groups (Table 2). Moreover, coinfections caused by influenza and 2 or more ORVs were also more frequent in young children (n = 53; 26.6%), but had a similar frequency in children (n = 15; 13.8%) and the remaining age groups. The virus detected most frequently in coinfection with influenza differed between age groups, with bocavirus being the most frequently detected in young children (0–4 years), rhinovirus/enterovirus the most frequent in children (5–14 years) and young adults (15–39 years), hCoV in adults (40–64 years) and RSV in the elderly (≥65 years).
Number and percentage of influenza-positive samples with detected coinfection by 1 ORV or 2 or more ORV in each category. The table also shows the virus detected most frequently in coinfection with influenza for each variable under study.
| Factor | Total samples, n | Samples with no coinfection, n (%) | Samples with coinfection, n (%) | Coinfection with 1 virus, n (% of coinfected) | Coinfection with ≥ 2 viruses, n (% of coinfected) | Virus most frequently detected in coinfection, n (% of coinfected) | |
|---|---|---|---|---|---|---|---|
| Age | 0–4 years | 647 | 448 (69.2%) | 199 (30.8%) | 146 (73.4%) | 53 (26.6%) | Bocavirus, 67 (33.6%) |
| 5–14 years | 526 | 417 (79.3%) | 109 (20.7%) | 94 (86.2%) | 15 (13.8%) | Entero/rhinovirus, 34 (31.2%) | |
| 15–39 years | 382 | 337 (88.2%) | 45 (11.8%) | 37 (82.2%) | 8 (17.8%) | Coronavirus, 13 (28.9%) | |
| 40–64 years | 757 | 663 (87.6%) | 94 (12.4%) | 79 (84.0%) | 15 (16.0%) | Entero/rhinovirus, 26 (27.7%) | |
| ≥65 years | 1311 | 1113 (84.9%) | 198 (15.1%) | 164 (82.8%) | 34 (17.2%) | RSV, 62 (31.3%) | |
| Sex | Male | 1854 | 1500 (80.9%) | 354 (19.1%) | 301 (85.0%) | 50 (15.0%) | Entero/rhinovirus, 96 (27.1%) |
| Female | 1769 | 1478 (83.6%) | 291 (16.4%) | 221 (76.0%) | 69 (24.0%) | RSV, 77 (26.5%) | |
| Type of patient | Inpatient | 2681 | 2198 (82.0%) | 483 (18.0%) | 375 (77.6%) | 108 (22.4%) | RSV, 127 (26.3%) |
| OP Sentinel | 942 | 780 (82.8%) | 162 (17.2%) | 137 (84.6%) | 25 (15.4%) | Entero/rhinovirus, 57 (35.1%) | |
| Influenza type/subtype | A(H3N2) | 1808 | 1452 (80.3%) | 356 (19.7%) | 289 (81.2%) | 67 (18.8%) | Entero/rhinovirus, 102 (28.7%) |
| A(H1N1)pdm09 | 971 | 819 (84.4%) | 152 (15.6%) | 120 (78.9%) | 32 (21.1%) | Entero//rhinovirus, 35 (23.0%) | |
| B | 844 | 707 (83.8%) | 137 (16.2%) | 115 (83.9%) | 22 (16.1%) | RSV, 33 (24.1%) | |
OP, outpatient; RSV, respiratory syncytial virus.
The proportion of coinfection of influenza and ORVs was higher in male patients (n = 354; 19.1%) compared to female patients (n = 291; 16.4%), although there was a higher percentage of female patients with coinfections of influenza and 2 or more ORVs (n = 69; 24.0%) compared to male patients. The frequency of coinfection of influenza and ORVs was similar in hospitalized patients (n = 483; 18.0%) and sentinel centre patients (n = 162; 17.2%), although the proportion of coinfections involving 2 or more ORVs was higher in inpatients (n = 108; 22.4%). Influenza A(H3N2) virus was the subtype involved most frequently in coinfections with ORVs (n = 356; 19.7%), while the A(H1N1)pdm09 subtype was involved most frequently in cases of coinfection with 2 or more ORVs (n = 32; 21.1%).
Analysis of factors associated with coinfection of influenza and other respiratory virusesTo analyse the variables associated with coinfection between influenza and ORVs, we performed a multivariate analysis (logBin) including age, sex, the type of patient (hospitalized/sentinel) and the type/subtype of influenza detected as independent variables. In this analysis, we focused on 3 different approximations; the first (A), to find out the variables associated with a higher probability of detecting coinfection of influenza and at least 1 ORV; the second (B), to find out the variables associated with a higher probability of detecting coinfection of influenza and only 1 ORV, and the third (C) to find out the variables associated with a higher probability of detecting coinfection of influenza and 2 or more ORVs.
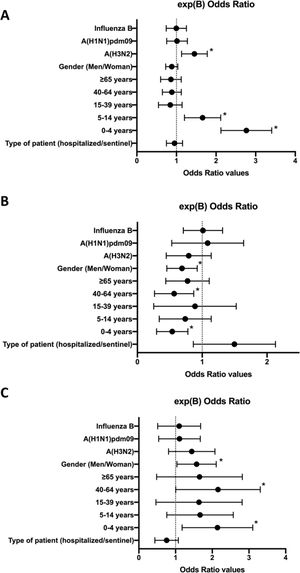
The multivariate analysis was significant for the three approximations (P < .05). In approximation (A), we found a higher probability of detecting coinfection of influenza and at least 1 ORV in case of infection by the A(H3N2) subtype (OR = 1.4; 95% confidence interval [CI], 1.14–1.79), in young children aged 0-4 years (OR = 2.7; 95% CI, 2.2–3.4), and in children aged 5–14 years (OR = 1.6; 95% CI, 1.2–2.1) (P < .05) (R2 = 0.03–0.05) (Fig. 1). In approximation (B), we found a lower probability of detection of coinfection of influenza and only 1 ORV in young children aged 0–4 years (OR = 0.5; 95% CI, 0.32–0.8), in adults aged 40-64 years (OR = 0.5; 95% CI, 0.3–0.9), and in female patients (OR = 0.7; 95% CI, 0.5–0.9) (P < .05) (R2 = 0.03–0.04). On the other hand, in approximation (C), we found a higher probability of detecting coinfection of influenza and 2 or more ORVs in young children aged 0–4 years (OR = 2.0; 95% CI, 1.3–3.2), in adults aged 40–64 years (OR = 2.0; 95% CI, 1.1–3.4), and in female patients (OR = 1.5; 95% CI, 1.1–2.1) (P < .05) (R2 = 0.03–0.4). We did not find any other differences in other variables in these 3 approximations.
Forest plots of the multivariate analysis (logBin) for each of the 3 approximations, expressing the exp(B) 95% confidence interval values for each study variable as odds ratios. A, Probability of coinfection by influenza and at least one ORV; B, Probability of coinfection by influenza and only 1 ORV; C, Probability of coinfection by influenza and 2 or more ORVs. *; P < .05.
In many cases, clinicians do not consider the potential involvement of more than 1 virus in respiratory infections.19 However, the detection of various respiratory viruses in coinfection with influenza is frequent during epidemic seasons when the diagnosis is made by means of molecular multiplex techniques,19,20 so this possibility should be taken into account. Our study shows that coinfections of influenza and ORVs were present in approximately 2 out of 10 patients (17.8%) with a positive test for influenza, demonstrating that such coinfections are relatively frequent during influenza epidemics. Out of all coinfected patients, most (76.0%) had coinfection with influenza and only 1 ORV, and 24.0% had coinfection by influenza and 2 or more ORVs. Other studies have found similar proportions of coinfection (4%–33%).20–25
Our findings show that the respiratory viruses more frequently involved in coinfections of influenza were rhinovirus/enterovirus (24.0%), RSV (19.1%), bocavirus (19.1%) and human coronavirus (18.3%). These respiratory viruses are among the most prevalent throughout the year, especially in the winter months of the Northern Hemisphere,1 and also have been found in similar percentages in other studies.24 The PCR protocol used in our study cannot differentiate between rhinovirus and enterovirus, so the proportion of each viral genus in these respiratory infections cannot be established with certainty. However, a previous study conducted by our group found that rhinoviruses were involved in more than 90% of those samples, so that proportion was likely to be maintained in the study presented here.26
The respiratory virus detected most frequently in coinfection with influenza varied by age group. Bocavirus was the most frequently detected in young children (0–4 years), rhinovirus/enterovirus in children (5–14 years) and young adults (15–39 years), hCoV in adults (40–64 years) and RSV in elderly patients aged 65 or more years. Some previous studies have found that rhinovirus was the virus detected most frequently in coinfections with influenza and with ORVs.12 Rhinoviruses play a relevant clinical role in both upper and lower respiratory tract diseases, and they are the leading cause of pneumonia in adults27 and the second leading cause in children.28 However, previous studies conducted in Spain have shown that RSV is the virus most frequently co-detected with influenza virus, especially in young individuals.24 Notwithstanding, in our study we found the highest proportion of coinfection with RSV in the elderly group, which suggests that this virus must be considered in adults, and not only in children.29
On the other hand, in 2020, the SARS-CoV-2 pandemic has highlighted the importance of hCoV in respiratory illness. Our results showed that hCoVs were the viruses most frequently detected in coinfection with influenza in adults (40-64 years), which is one of the age groups most affected by COVID-19 in the pandemic.30,31 The potential concurrence of influenza, ORV and SARS-CoV-2 must be taken into account in upcoming years, especially in the most vulnerable groups. Although our findings show that hCoVs are frequently co-detected with influenza in adults, we were not able to evaluate the potential involvement of SARS-CoV-2. However, some studies have been published by now that analysed coinfection by influenza and SARS-CoV-2 and suggest these coinfections may be common and be associated with increased severity in some cases.32,33
Our results showed a clear association between coinfections and age. The multivariate analysis showed that detection of coinfection by influenza and ORVs was 2.7 times more frequent in young children and 1.6 times more frequent in children. But also, coinfection seemed to be associated with the involved subtype of the influenza virus, as coinfections with ORVs were 1.4 times more frequent in patients infected by the A(H3N2) subtype.
There are several possible explanations for the higher probability of detection of coinfection in the youngest patients. One is that children have an immature immune system compared to adults, increasing the probability of infection by several respiratory viruses at the same time.34–36 Also, the greater interaction of children in schools also increases the opportunity of exposure to different viruses.13,37 The higher proportion of the A(H3N2) subtype in cases of coinfection could be explained by the greater virulence of this subtype compared to other influenza viruses.38,39 This greater virulence can cause changes in epithelial cells, weakening the immune response and facilitating infection by ORVs and bacteria.40 However, this issue requires further investigation.
Our results also showed that the prevalence of coinfection by 2 or more ORVs was two-fold in young children, two-fold in adults and 1.5-fold in female patients. Coinfections by 2 or more ORVs were particularly frequent adult women (40–64 years). One explanation could be the increased exposure of women to children, who are also affected also by multiple coinfections, due to their higher involvement in child care compared to men.41 In fact, the World Health Organization suggests that this factor is more important than differences in immunity related to sex.42 However, we did not have enough samples to test this issue thoroughly, so further research must be conducted in the future. On the other hand, the type of patient (hospitalized or sentinel) did not seem to be a factor associated with the probability of detection of coinfection by influenza and ORVs.
One of the main limitations of this study was its retrospective design, as we were unable to assess the outcomes of the included patients, such as duration of disease, admission to the ICU and mortality. Further studies are required to analyse outcomes and their association with the presence of coinfection by influenza and ORVs in detail. One of the main strengths of our study is that it covered a large number of influenza seasons, which compensated the variability intrinsic in each season and provided more accurate information on the factors that are truly involved in coinfection, such as age.
In summary, our study found that coinfections by influenza and ORVs were detected in nearly 2 out of 10 laboratory-confirmed influenza cases, and that such coinfections were more frequent in young children (0–4 years), children (5–14 years) and patients infected by the A(H3N2) influenza subtype. The virus detected most frequently in coinfection with influenza varied between age groups, with hCoV being the most frequently detected in adults (40–64 years). While sex was not associated with a higher incidence of coinfection, there was a higher frequency of coinfection by influenza and 2 or more ORVs in female patients, probably due to the greater involvement of mothers in caring for children, as children had the highest rates of coinfection, both by a single and by multiple ORVs. These results could be useful to guide the use of multiplex PCR for diagnosis of respiratory viruses during the influenza season in laboratories with low resources.
Please cite this article as: Sanz I, Perez D, Rojo S, Domínguez-Gil M, de Lejarazu RO, Eiros JM. Las coinfecciones entre gripe y otros virus respiratorios están asociadas a los niños. An Pediatr (Barc). 2022;96:334–341.