We present 3 cases of cerebral air embolism in newborn infants.
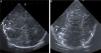
Case 1Girl born preterm (PT) at 24 weeks’ gestation with a birth weight of 764 g. After birth, the patient required level IV advanced neonatal resuscitation with endotracheal intubation, invasive mechanical ventilation and umbilical vein catheterization. At 5 days post birth, she exhibited sudden deterioration with desaturation, bradycardia, marked irritability and uncoordinated movements requiring high-frequency ventilation and sedation with morphine. The amplitude-integrated electroencephalogram (aEEG) evinced convulsive seizures that coincided with sucking movements, which resolved after administration of 2 boluses of phenobarbital. A transfontanellar ultrasound examination revealed several hyperechoic birefringent features in the periventricular region compatible with air embolism (Fig. 1). The follow-up scan at 24 h revealed a decrease in the number of hyperechoic features, with full resolution of these sonographic abnormalities in subsequent days. The patient died at 40 weeks of postmenstrual age of necrotising enterocolitis and persistent sepsis.
Ultrasound sagittal (A and B) and coronal (C) views showing multiple hyperechoic, birefringent spots and lines in the deep periventricular white matter on both sides of the brain (arrows in A and C) and right caudothalamic groove (arrow in B) that could be attributed to cerebral air embolism.
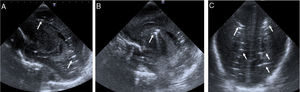
Male neonate born at term that had bradycardia during the second stage of labour. He required level IV advanced resuscitation with intubation and mechanical ventilation due to sustained hypoxaemia. Postnatal diagnosis of hypoplastic left heart. The patient underwent catheterization of umbilical vessels for monitoring and delivery of inotropic agents. Due to severe hypoxaemia, the patient was placed under extracorporeal membrane oxygenation (ECMO) and underwent emergency surgery. The transfontanellar ultrasound scan performed before initiation of ECMO revealed diffuse cerebral oedema with hyperechoic spots in the cortical region and bilateral birefringent hyperechoic foci in the parieto-occipital region that could have been produced by small air bubbles (Fig. 2). In the follow-up ultrasound scan at 24 h, the only feature that remained was the cortical hyperechogenicity. The patient died 11 days post birth due to severe hypoxic-ischaemic encephalopathy.
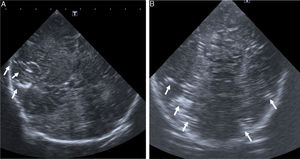
Case 3Boy born preterm at 34 weeks with a birth weight of 1740 g, delivered by emergency Caesarean section due to foetal bradycardia. He required level IV advanced resuscitation with intubation, conventional mechanical ventilation, and umbilical catheter placement for monitoring and delivery of blood products. The transfontanellar ultrasound scan at 3 h post birth showed hyperechoic bands arranged in the shape of a semi-oval outline in the periatrial white matter of the right hemisphere, suggestive of air embolism (Fig. 3). The patient exhibited gradual clinical improvement in the continuous aEEG monitoring, with a normal neurologic assessment. The follow-up ultrasound scan at 24 h was normal. The patient was discharged at 9 days post birth with age-appropriate psychomotor development.
Air embolisms are produced by the traumatic or iatrogenic entry of air in the venous or arterial circulation as a result of surgery, traumatic injury, intravascular procedures, invasive mechanical ventilation,1 non-invasive mechanical ventilation, cardiopulmonary resuscitation2 or even necrotising enterocolitis, and remains an underdiagnosed condition. It may affect different areas, including pulmonary circulation, the heart or the brain, and can even trigger a systemic inflammatory response.1
Cerebral air embolisms may originate in the venous pulmonary circulation due to the presence of a bronchovenous fistula or a vascular lesion secondary to barotrauma.3 There is a higher risk in the paediatric population due to the immaturity of the lungs, with the greatest mortality found in preterm infants.4 Entry of air into the brain may also result from paradoxical flow induced by a patent foramen ovale1 or retrograde ascent of air bubbles in the opposite direction of the venous flow.5
In the case series presented here, all infants had a history of umbilical catheter placement and handling, which may have been the cause of the embolism.
The clinical manifestations of cerebral air embolism vary and depend on the site of occlusion, the size of the bubbles, the speed of entry, the position of the patient and the health status of the patient,5 ranging from trivial to catastrophic.1 They may range from transient neurologic symptoms, such as convulsive seizures (case 1) to sudden loss of consciousness or death. It is of utmost importance that this entity is suspected in patients exhibiting an abrupt worsening following a compatible medical procedure.
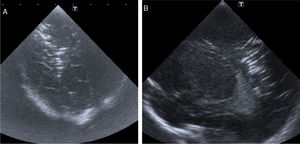
As described in our case series and other articles, a transfontanellar ultrasound scan can contribute to an early diagnosis, in some cases resulting from a chance finding,6 as was the case in 2 of our patients. We used a Philips Affiniti® 70G ultrasound machine with a cephalic imaging C8-5 transducer (frequency range, 5–8 Hz). In other age groups, the gold standard for diagnosis would be computed tomography,6 which should be performed in the acute phase,6,7 as imaging tests in subsequent stages may find features corresponding to resolution of the embolism.
Treatment in symptomatic patients consists of hyperbaric oxygen therapy or high-flow oxygen therapy1,8,9 and placing the patient in the Trendelenburg position,1,9 although at present there is no protocol for management of cerebral air embolism in newborns. Oxygen delivery is not only important for management of hypoxaemia, but also in reducing the size of the air bubbles by establishing a diffusion gradient that favours elimination of the gas.10
Since the consequences of cerebral air embolism can be dire and there is no standardised treatment protocol for the neonatal population, the main strategy is currently prevention1 and the optimization of procedures that may cause iatrogenic problems, in addition to including cerebral air embolism in the differential diagnosis of patients that exhibit sudden worsening with or without neurological manifestations.
Please cite this article as: Pérez A, Gregorio R, Gómez P, Ruiz Y, Sánchez-Luna M. Embolismo aéreo cerebral en neonatos. An Pediatr (Barc). 2020;93:54–57.