Enteroviruses (EV) and human parechoviruses (HPeV) are RNA viruses from the Picornaviridae family that are very prevalent in the paediatric population, causing disease in various forms.1–4 Febrile seizures are convulsions associated with fever in neurologically healthy infants and children (age 6 months to 5 years) without a history of febrile seizures and in the absence of central nervous system (CNS) infection.5 The potential involvement of EV and HPeV in febrile seizures during epidemic seasons is not known. Previous studies performed in children with CNS infections have found a high frequency of convulsive seizures in this context, such as the study performed by Karsch et al., who described seizures in 8 out of 12 children aged less than 4 years in whom HPeV had been detected in cerebrospinal fluid (CSF).6 However, the potential association between infection by these viruses and febrile seizures in healthy children has not been investigated in depth, which led us to conduct a pilot study on the subject.
The study included children aged 6 months to 5 years without neurologic disease that presented with febrile seizures to the paediatric emergency department of a tertiary care hospital in Madrid between April 2019 and April 2020, a year when there was evidence of circulation of EV and HPeV based on data from the department of microbiology of the hospital. Nasopharyngeal or rectal swabs were collected to perform polymerase chain reaction (PCR) tests for detection of EV and HPeV. In patients who underwent lumbar puncture, PCR testing for detection of these viruses was also performed in CSF. We documented the development of convulsions in each patient after the initial visit to the emergency department for a 6-month follow-up period after the end of the recruitment period. The statistical analysis was performed with the software IBM® SPSS® Statistics 20.
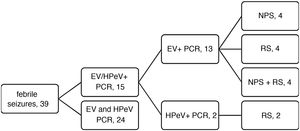
We analysed data for a total of 39 patients, and the PCR test was positive for EV or HPeV in 15 (38.5%; 13/39 for EV and 2/39 for HPeV). Fig. 1 and Table 1 summarise the main results of the study.
Clinical and laboratory characteristics, treatment and outcomes and the 39 patients with febrile seizures by study group.
| EV+ n = 13 | HPeV+ n = 2 | EV– and HPeV– n = 24 | |
|---|---|---|---|
| PCR | 13 (4 NPS, 2 RS, 7 NPS + RS) | 2 (RS) | 24 (–): 1 streptococcal AP, 1 AGE due to Yersinia, 2 RSV, 2 influenza A, 1 influenza B |
| Sex | 9 male, 4 female | 2 female | 14 male, 10 female |
| Age (median, IQR) | 2.09 years (1.75−2.75) | 8 months and 20 months | 1.72 years (1.13−2.77) |
| History of febrile seizures | 5 | 0 | 4 |
| Time from fever onset to visit (median, IQR) | 12 h (5.5−24) | 6 h and 1 h | 6 h (2−12) |
| Maximum temperature (median, IQR) | 39.2 °C (38.15−39.85) | 40 °C and 39 °C | 38.9 °C (38.4−39.3) |
| Associated clinical features | URTI, 9; nonspecific fever, 2; AOM, 1; AGE with status ep., 1 | URTI | nonspecific fever, 11; URTI, 9; AOM, 3 (1 with encephalitis); AGE, 1 |
| Type of seizure | Generalised tonic-clonic, 8 | Generalised tonic-clonic | Generalised tonic-clonic, 20 |
| Stiffness/hypertonia, 4 | Stiffness/hypertonia, 3 | ||
| Absence seizures and facial twitching with cyanosis, 1 | Absence seizures and facial twitching with cyanosis, 1 | ||
| Duration of seizures (median, IQR) | 2 min (1−4.5) | 4 min and 35 min | 1.5 min (1−5) |
| Recurrent seizures in ED (<24 h) | 0 | 1 | 1 |
| Postictal state | 100% | 100% | 100% |
| Blood tests, mean (SD) | |||
| WBC (cells/mm3) | 12.93 (5.77) | 16.59 (5.25) | 12.51 (6.4) |
| NC (cells/mm3) | 9.46 (4.7) | 9.35 (0.73) | 8.5 (4.8) |
| CPR (mg/L) | 36.19 (25.35) | 41.5 (30.55) | 26.85 (34.46) |
| PCT (ng/mL) | 0.57 (0.62) | 2.52 (0.18) | 0.45 (0.46) |
| CSF protein | 15.2 | 10.95 (3.18) | 17.9 (1.8) |
| WBC in CSF | 0 | 1.5 (2.12) | 2.75 (3.77) |
| Lumbar puncture | 1 (negative bacterial culture, EV and HPeV PCR) | 2 (negative bacterial culture, EV and HPeV PCR) | 4 (negative bacterial culture, EV and HPeV PCR) |
| EEG | 1 (normal) | 1 (normal) | 4 (normal) |
| Head CT | 1 (normal) | 1 (normal) | 4 (normal) |
| Length (median, IQR) | 3.5 h (2.75−5.5) | 7.5 h and 0.5 h | 7 h (3−9.75) |
| Admission | 2 (1 to ward and 1 to PICU due to status ep.) | 2 (1 to ward and 1 to PICU due to febrile status ep.) | 8 (7 to ward, 1 to PICU due to status ep.) |
| Length of stay | 5 days and 1 day | 2 días y 5 días | Median, 3.5 days (IQR, 2−4.75) |
| Treatment | Antibiotherapy, 3 | Antibiotherapy, 2 | Antibiotherapy, 7 |
| Acyclovir, 1 | Levetiracetam, 1 | Acyclovir, 1 | |
| Levetiracetam, 1 | Levetiracetam, 1 | ||
| Oxcarbazepine, 1 | |||
| Outcome | Favourable | Favourable | Favourable |
| Seizures in follow-up | 3 | 0 | 5 |
AGE, acute gastroenteritis; AOM, acute otitis media; AP, acute pharyngitis; CPR, C-reactive protein; CSF, cerebrospinal fluid; ED, emergency department; EEG, electroencephalogram; EV, enterovirus; HPeV: parechovirus; IQR, interquartile range; NC, neutrophil count; PCT, procalcitonin; PICU, paediatric intensive care unit; RSV, respiratory syncytial virus; SD, standard deviation; URTI, upper respiratory tract infection; WBC, white blood cell count.
When we compared the groups of patients, we did not find statistically significant differences in the clinical and laboratory characteristics between the children with a positive test result for the viruses under study (overall group with a positive PCR test for EV and/or HPeV) and those with negative PCR results. We only found differences in the body temperature and the CSF protein concentration in patients with a positive EV or HPeV PCR test (P = .024 and P = .035, respectively). On the other hand, when we compared specifically the group of patients with a positive EV PCR test (n = 13) to the group with a positive HPeV PCR test (n = 2) we found differences in procalcitonin levels (higher in the HPeV + group: mean, 25 ng/mL; P = .015). In addition, 100% of children with a positive PCR test for HPeV were admitted to hospital (P = .012), 100% received antibiotherapy (P = .032), with a higher frequency in this group of diagnosis of severe disease (P = .014), including recurrent seizures during the stay in the emergency department (n = 1) or status epilepticus (n = 1). In contrast, in the group of patients that tested positive for EV, only 1 patient (7.7%) experienced recurrent seizures during the stay in the emergency department. These findings must be interpreted with caution due to the small sample size, especially in the group of patients with a positive PCR test for HPeV, but they seem to corroborate the greater clinical severity of HPeV infections. On this particular, we ought to highlight the application of molecular techniques, which allow early diagnosis of these viral agents and therefore optimization of treatment. Another aspect worth mentioning is that in the 2 cases in our study in which HPeV was detected, it was in rectal swab samples, although the patients presented with respiratory symptoms, and not in CSF specimens, in which the virus has been detected more readily in other studies.6 On the other hand el 38.5% of the patients that tested positive for EV had experienced febrile seizures before. This may suggest that seizures may have to do with an individual predisposition rather than a specific infection. Based on this case series, it does not appear that the development of convulsive seizures in the context of EV or HPV infection is associated with an increased risk of recurring seizures compared to other aetiologies, although the follow-up was short, and the results should be interpreted with caution. All the patients had favourable outcomes without neurologic sequelae.
To conclude, in the sample under study, 38.5% of the cases of febrile seizures were associated with infection by EV or HPeV (33.3% and 5.1%, respectively), so these viruses should be taken into account as potential triggers of febrile convulsive seizures. The main limitation of our study is that it was conducted in a single centre, so that the sample size was small, and that it did not include every patient that met the inclusion criteria. Another important limitation is that few patients underwent lumbar puncture (n = 7), especially in the group positive for EV (n = 1), which limits the validity of our findings in this regard. In addition, the viruses were identified in nasopharyngeal and/or rectal swab samples, so it was not possible to establish with certainty that they were the aetiological agents involved in the infection, as they were not detected in CSF or blood. On the other hand, the duration of follow-up was brief. We consider that more studies with larger samples and longer follow-up are required to establish the course and recurrence of febrile seizures in patients with infection by EV and HPeV.
Please cite this article as: García Sánchez P, de Ceano-Vivas la Calle M, Romero Gómez MP, García Bujalance S, Calvo Rey C. Asociacion de crisis febriles e infección por enterovirus y parechovirus humano en urgencias pediátricas: estudio piloto. An Pediatr (Barc). 2022;96:457–459.