Health care-associated infections (HAIs) contribute to morbidity and mortality and to the dissemination of multidrug-resistant organisms. Children admitted to the intensive care unit undergo invasive procedures that increase their risk of developing HAIs and sepsis. The aim of the study was to analyse factors associated with mortality due to sepsis arising from HAIs.
Patients and methodsWe conducted a case-control study in a 7-bed multipurpose paediatric intensive care unit in a tertiary care teaching hospital. The sample consisted of 90 children admitted between January 2014 and December 2018. The case group consisted of patients who died from sepsis associated with the main health care-associated infections; the control group consisted of patients who survived sepsis associated with the same infections.
ResultsDeath was associated with age less than or equal to 12 months, presence of comorbidity, congenital disease, recurrent ventilator-associated pneumonia and septic shock. In the multiple regression analysis, heart disease (OR, 12.48; CI 2.55–60.93; P = .002), infection by carbapenem-resistant bacteria (OR, 31.51; CI 4.01–247.25; P = .001), cancer (OR, 58.23; CI 4.54–746.27; P = .002), and treatment with adrenaline (OR, 13.14; CI 1.35–128.02; P = .003) continued to be significantly associated with death.
ConclusionsHospital sepsis secondary to carbapenem-resistant bacteria contributed to a high mortality rate in this cohort. Children with heart disease or neoplasia or who needed vasopressor drugs had poorer outcomes.
Las infecciones relacionadas con la asistencia sanitaria (IRAS) contribuyen a la morbimortalidad y a la diseminación de organismos multirresistentes. Los niños ingresados en la unidad de cuidados intensivos son sometidos a procedimientos invasivos que aumentan su riesgo de desarrollar IRAS y sepsis. El objetivo de este estudio fue analizar los factores asociados a la letalidad por sepsis derivada de IRAS.
Pacientes y métodosEstudio de casos y controles, en una unidad de cuidados intensivos pediátricos polivalente de 7 camas de un hospital universitario de tercer nivel. La muestra consistió en 90 niños ingresados entre enero de 2014 y diciembre de 2018. Los casos se definieron como fallecimientos por sepsis asociada a infecciones asistenciales principales; los controles fueron los pacientes supervivientes que presentaron sepsis asociada a las mismas infecciones.
ResultadosLa muerte se asoció a edad menor o igual a 12 meses, presencia de comorbilidad, enfermedad congénita, neumonía recurrente asociada a ventilación mecánica y shock séptico. En el análisis múltiple, la cardiopatía (OR 12,48; IC 2,55–60,93; p = 0,002), la infección por bacterias resistentes a carbapenémicos (OR 31,51; IC 4,01–247,25; p = 0,001), el cáncer (OR 58,23; IC 4,54–746,27; p = 0,002) y el uso de adrenalina (OR 13,14; IC 1,35–128,02; p = 0,003) siguieron asociados a la muerte.
ConclusionesLa sepsis hospitalaria secundaria a bacterias resistentes a carbapenémicos contribuyó a una elevada tasa de mortalidad. Los niños con cardiopatía o neoplasia o que necesitaron fármacos vasopresores tuvieron peores desenlaces.
Healthcare-associated infections (HAIs) are among the most frequent adverse events and constitute a public health problem that contributes to morbidity and mortality, length and costs of hospitalization and the selection and spread of multidrug-resistant organisms (MDROs). It is estimated that for every 100 patients hospitalized in developed countries, seven will develop a HAI; in developing countries, this number rises to 10.1 The costs of hospitalization for children who develop HAIs can be up to 4 times higher compared to children who do not develop this complication.2 Health care-associated infections are more frequent in neonatal and paediatric intensive care units (PICUs) compared to other paediatric settings. A multicentre European study in children showed an overall prevalence of HAIs of 4.5%, with 15.5% in PICUs.3
Health care-associated infections are often related to invasive devices, with the most common instances being central venous catheter bloodstream infection (BSI), ventilator-associated pneumonia (VAP) and urinary tract infection (UTI) associated with prolonged use of a urinary catheter. Children admitted to the ICU undergo invasive procedures, such as tracheal intubation, venous and urinary catheter insertion, chest drainage and surgical procedures, that increase their risk of developing HAIs and sepsis, both of which are associated with an increased mortality.4,5 Paediatric sepsis is a frequent cause of death in children, particularly in developing countries. Nevertheless, due to the nonspecificity of the clinical manifestations in different age groups, it is still underreported.6,7 Hospital-acquired sepsis, that is, sepsis associated with the length of stay or to procedures performed in hospital, may account for 10%–20% of cases of sepsis and is associated with a higher mortality compared to community-acquired sepsis.8
An analysis of the mortality due to sepsis in Brazil using data from the Unified Health Care System from 2006 to 2015 found a mean mortality of 46.3% overall, of 64.5% in the ICU and of 13.6% in group aged 0–17 years.9
In light of the above, we conducted a study to analyse factors associated with mortality due to sepsis resulting from HAIs in children admitted to the ICU of a public university hospital in southern Brazil.
Material and methodsWe conducted a case-control study by collecting data from health records, the department of records and archives and the HAI notification forms of the Hospital Infection Control Committee of the teaching hospital affiliated to the State University of Londrina in the State of Paraná, South Brazil. The project was approved by the Ethics Committee for Research Involving Human Beings of the university (certificate of ethical approval no. 28068119.6.0000.5231; opinion no. 3 991 033 of April 26, 2020).
The sample included all children aged 29 days to 11 years, 11 months and 29 days admitted to the paediatric intensive care unit (PICU) of the University Hospital of Londrina between January 2014 and December 2018 and who developed BSI, pneumonia, or UTI associated with invasive devices and experienced progression to sepsis based on the HAI notification system of the Hospital Infection Control Committee (HICC). We excluded patients receiving palliative care.
The PICU of the studied hospital is a high-complexity referral unit accredited by the public health system of Brazil and serving patients from 250 municipalities in Paraná and 100 municipalities in other states. During the study period, the PICU had seven beds for medical and surgical patients, providing an average of 159.3 patient days of care based on the monthly calculation by the HICC. We defined case as death due to sepsis associated with VAP, UTI, or BSI; the controls were patients who developed sepsis in association with the same infections but did not die.
The independent variables under study were sex, age, presence of comorbidity, diagnosis at admission, type of HAI (BSI, VAP or UTI), presence of two or more HAIs, length of hospital stay (days), length of PICU stay (days), days of therapy with antimicrobials, progression to septic shock, use of catecholamine vasopressors (norepinephrine, adrenaline), serum lactate level and C-reactive protein level.
When it came to the aetiological agent and the antimicrobial resistance profile, we classified pathogens as carbapenem-resistant (CR) gram-negative bacilli and multidrug-resistant (MDR) microorganisms (defined as gram-negative bacilli resistant to third- or fourth-generation cephalosporins, extended-spectrum beta-lactamase-producing gram-negative bacilli and oxacillin-resistant Staphylococcus spp).
We analysed the association between sepsis and mortality according to the information recorded in the HAI notification forms of the Hospital Infection Control Committee. For cases involving more than one infection that progressed to sepsis, we attributed the death in the health records according to the evaluation of the death by the medical team of the Hospital Infection Control Committee.
We collected data on the laboratory tests performed between 24 h before or 24 h after the initial detection of the HAI; if more than one infection was present in the same patient, we used the laboratory results corresponding to the last detected HAI. When it came to cultures of samples from infectious sites, we considered the samples collected in the same time interval.
The medical team of the Hospital Infection Control Committee applied the criteria of the National Health Surveillance Agency for diagnosis of HAI. The criteria for sepsis and septic shock applied for diagnosis were those established in International Paediatric Sepsis Consensus Conference guidelines,10 and the Surviving Sepsis Campaign11 guidelines were used to guide treatment. All children received empiric antibiotherapy followed by treatment based on the antimicrobial susceptibility profile shortly after this result became available.
We entered the data in Microsoft Excel and analysed them with the Statistical Package for the Social Sciences (SPSS), version 20.0. We expressed categorical variables as absolute and relative frequencies and continuous variables as median and interquartile range (IQR). To evaluate associations between categorical variables and mortality, we used the χ2 test or Fisher exact test, as applicable. The association between continuous variables and mortality was analysed by means of simple logistic regression. We performed a multiple logistic regression analysis selecting those variables with a P value of less than 0.20 in the bivariate analysis. The regression model was fitted using the following tests: Omnibus, Nagelkerke R2, Hosmer-Lemeshow test, and residual diagnostics. We used stepwise forward selection to input variables into the model. The measure used to quantify the association was the odds ratio (OR) with its 95% confidence interval (CI). We set the level of significance at 5% (P < .05).
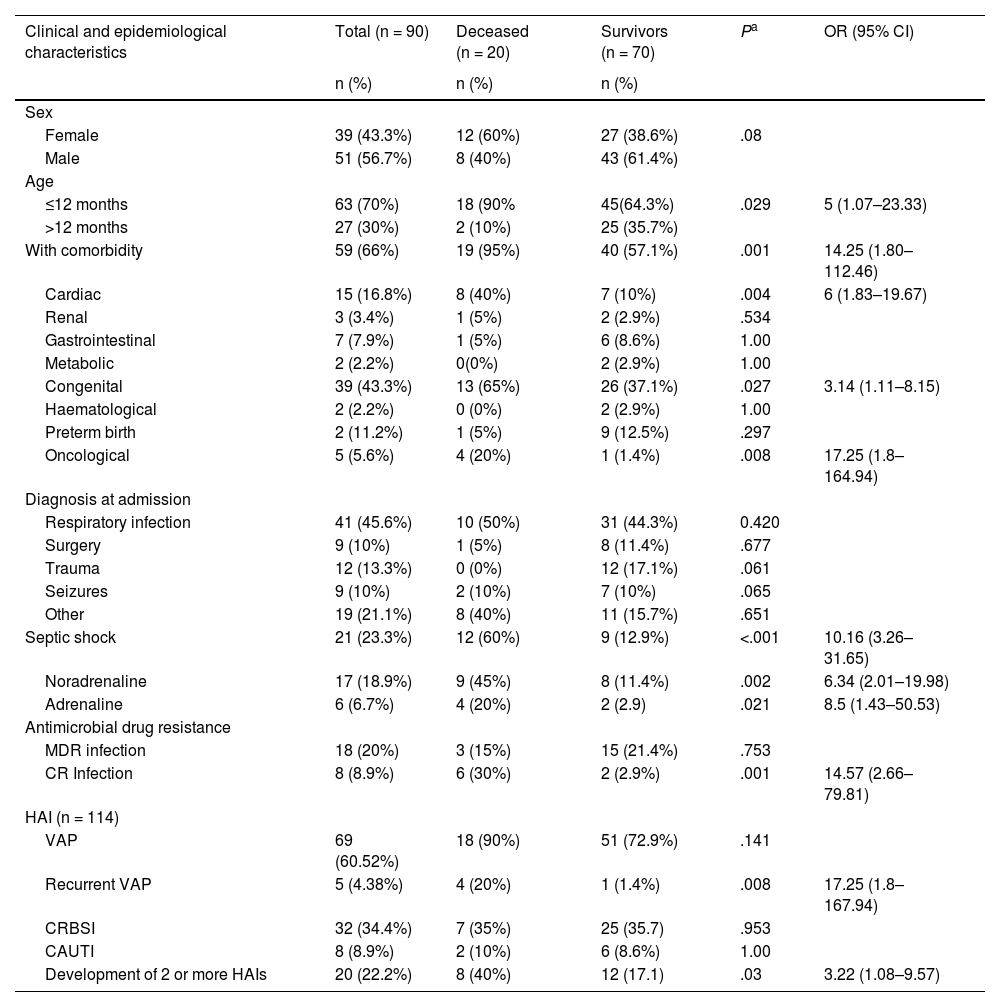
ResultsThe sample included 90 children with HAI and sepsis, with a predominance of patients of male sex, aged less than 12 months, with comorbidities and with a diagnosis of infectious disease at admission (Table 1). The median age was 10 months (IQR, 20.7), and the median length of stay in the PICU was 13.5 days (IQR, 20). Thirty percent of the children had infections by bacteria resistant to various antibiotics. Nearly one quarter of the children (23.3%) developed septic shock, and noradrenaline was the vasoactive drug used in 81% of these cases. The lethality rate of hospital-acquired sepsis was 22.2%. A total of 114 HAIs were detected, most frequently VAP, followed by BSI and UTI. We found no association between the type of HAI and mortality (Table 1).
Clinical and epidemiological characteristics of patients with hospital-acquired sepsis and their association with mortality.
| Clinical and epidemiological characteristics | Total (n = 90) | Deceased (n = 20) | Survivors (n = 70) | Pa | OR (95% CI) |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| Sex | |||||
| Female | 39 (43.3%) | 12 (60%) | 27 (38.6%) | .08 | |
| Male | 51 (56.7%) | 8 (40%) | 43 (61.4%) | ||
| Age | |||||
| ≤12 months | 63 (70%) | 18 (90% | 45(64.3%) | .029 | 5 (1.07–23.33) |
| >12 months | 27 (30%) | 2 (10%) | 25 (35.7%) | ||
| With comorbidity | 59 (66%) | 19 (95%) | 40 (57.1%) | .001 | 14.25 (1.80–112.46) |
| Cardiac | 15 (16.8%) | 8 (40%) | 7 (10%) | .004 | 6 (1.83–19.67) |
| Renal | 3 (3.4%) | 1 (5%) | 2 (2.9%) | .534 | |
| Gastrointestinal | 7 (7.9%) | 1 (5%) | 6 (8.6%) | 1.00 | |
| Metabolic | 2 (2.2%) | 0(0%) | 2 (2.9%) | 1.00 | |
| Congenital | 39 (43.3%) | 13 (65%) | 26 (37.1%) | .027 | 3.14 (1.11–8.15) |
| Haematological | 2 (2.2%) | 0 (0%) | 2 (2.9%) | 1.00 | |
| Preterm birth | 2 (11.2%) | 1 (5%) | 9 (12.5%) | .297 | |
| Oncological | 5 (5.6%) | 4 (20%) | 1 (1.4%) | .008 | 17.25 (1.8–164.94) |
| Diagnosis at admission | |||||
| Respiratory infection | 41 (45.6%) | 10 (50%) | 31 (44.3%) | 0.420 | |
| Surgery | 9 (10%) | 1 (5%) | 8 (11.4%) | .677 | |
| Trauma | 12 (13.3%) | 0 (0%) | 12 (17.1%) | .061 | |
| Seizures | 9 (10%) | 2 (10%) | 7 (10%) | .065 | |
| Other | 19 (21.1%) | 8 (40%) | 11 (15.7%) | .651 | |
| Septic shock | 21 (23.3%) | 12 (60%) | 9 (12.9%) | <.001 | 10.16 (3.26–31.65) |
| Noradrenaline | 17 (18.9%) | 9 (45%) | 8 (11.4%) | .002 | 6.34 (2.01–19.98) |
| Adrenaline | 6 (6.7%) | 4 (20%) | 2 (2.9) | .021 | 8.5 (1.43–50.53) |
| Antimicrobial drug resistance | |||||
| MDR infection | 18 (20%) | 3 (15%) | 15 (21.4%) | .753 | |
| CR Infection | 8 (8.9%) | 6 (30%) | 2 (2.9%) | .001 | 14.57 (2.66–79.81) |
| HAI (n = 114) | |||||
| VAP | 69 (60.52%) | 18 (90%) | 51 (72.9%) | .141 | |
| Recurrent VAP | 5 (4.38%) | 4 (20%) | 1 (1.4%) | .008 | 17.25 (1.8–167.94) |
| CRBSI | 32 (34.4%) | 7 (35%) | 25 (35.7) | .953 | |
| CAUTI | 8 (8.9%) | 2 (10%) | 6 (8.6%) | 1.00 | |
| Development of 2 or more HAIs | 20 (22.2%) | 8 (40%) | 12 (17.1) | .03 | 3.22 (1.08–9.57) |
CAUTI, catheter-associated urinary tract infection; CR, carbapenem-resistant; CRBSI, catheter-related bloodstream infection; HAI, health care-associated infection; MDR, multidrug-resistant; VAP, ventilator-associated pneumonia.
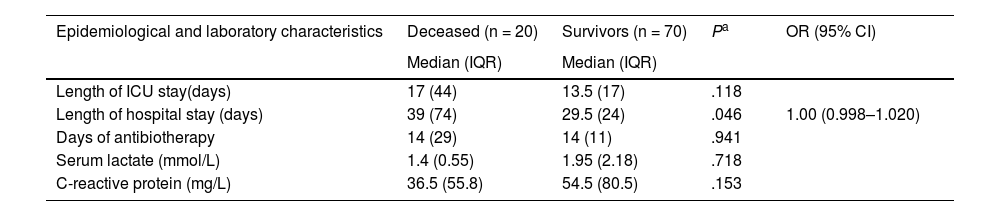
The duration of antibiotherapy in days was not associated with mortality (Table 2). Concerning the use of empirical antibiotherapy, VAP was most frequently treated with piperacillin/tazobactam and amikacin (62.3% of treatments), both for a median duration of 7 days (IQR, 3). When it came to BSIs, vancomycin was used in 43.9% of cases for a median duration of 10 days (IQR, 7), and when it came to UTIs, amikacin was used in 62.5% of the cases, with a median duration of 7 days (IQR, 3). The antibiotics used for empirical treatment in children with infections by microorganisms that turned out to be CR were amikacin in 4 (2 with CR strains susceptible to amikacin who lived, 1 with a CR strain susceptible to amikacin who died, and 1 with a CR strain also resistant to amikacin who died), carbapenem in 1, cefepime in 1, glycopeptide in 1 and polymyxin in 1 child, all 4 of who died despite adjustment of treatment based on the results of susceptibility testing. In all children with infection by a CR microorganism, the empirical antimicrobial treatment was switched to polymyxin, amikacin, tigecycline or a combination thereof as soon as the culture results became available. Therefore, the empirical treatment was appropriate in 4 children, of who 2 still died, and inappropriate in 4 patients, all of who died.
Length of stay, duration of antibiotic therapy and laboratory results of patients with hospital-acquired sepsis and their association with mortality.
| Epidemiological and laboratory characteristics | Deceased (n = 20) | Survivors (n = 70) | Pa | OR (95% CI) |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | |||
| Length of ICU stay(days) | 17 (44) | 13.5 (17) | .118 | |
| Length of hospital stay (days) | 39 (74) | 29.5 (24) | .046 | 1.00 (0.998–1.020) |
| Days of antibiotherapy | 14 (29) | 14 (11) | .941 | |
| Serum lactate (mmol/L) | 1.4 (0.55) | 1.95 (2.18) | .718 | |
| C-reactive protein (mg/L) | 36.5 (55.8) | 54.5 (80.5) | .153 |
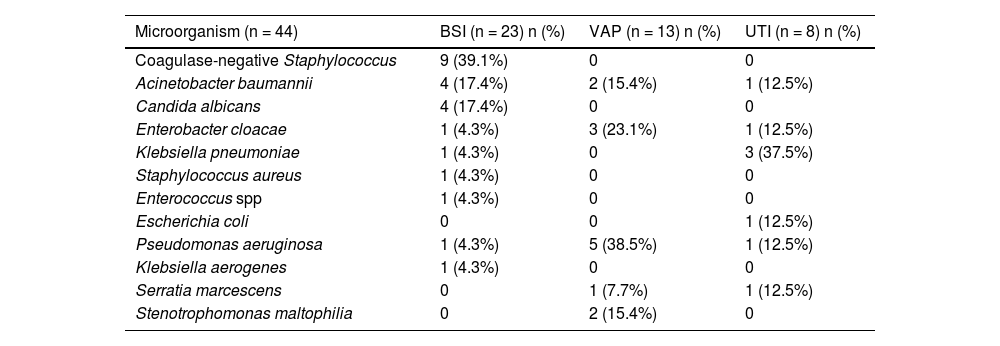
A microorganism was isolated in 38.5% of the analysed HAIs. Gram-negative bacilli were isolated most frequently, accounting for 65.9% of isolates. The aetiological agent was identified in 17.6% of the 74 cases of VAP and in 71.9%. of the 32 patients with BSI. Pseudomonas aeruginosa was the pathogen identified most frequently in cases of VAP. In the BSI group, isolation of gram-positive cocci was more frequent, accounting for 47.3% of the cases. The most frequently isolated pathogen in patients with UTI was Klebsiella pneumoniae (Table 3).
Aetiological agents involved in sepsis related to healthcare-associated infection.
| Microorganism (n = 44) | BSI (n = 23) n (%) | VAP (n = 13) n (%) | UTI (n = 8) n (%) |
|---|---|---|---|
| Coagulase-negative Staphylococcus | 9 (39.1%) | 0 | 0 |
| Acinetobacter baumannii | 4 (17.4%) | 2 (15.4%) | 1 (12.5%) |
| Candida albicans | 4 (17.4%) | 0 | 0 |
| Enterobacter cloacae | 1 (4.3%) | 3 (23.1%) | 1 (12.5%) |
| Klebsiella pneumoniae | 1 (4.3%) | 0 | 3 (37.5%) |
| Staphylococcus aureus | 1 (4.3%) | 0 | 0 |
| Enterococcus spp | 1 (4.3%) | 0 | 0 |
| Escherichia coli | 0 | 0 | 1 (12.5%) |
| Pseudomonas aeruginosa | 1 (4.3%) | 5 (38.5%) | 1 (12.5%) |
| Klebsiella aerogenes | 1 (4.3%) | 0 | 0 |
| Serratia marcescens | 0 | 1 (7.7%) | 1 (12.5%) |
| Stenotrophomonas maltophilia | 0 | 2 (15.4%) | 0 |
BSI, bloodstream infection; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
Of the total isolated microorganisms, 59.1% exhibited some resistance mechanism, out of which 18 (41%) were MDR organisms and 8 (18.2%) were CR. In the subset of CR bacteria, Acinetobacter baumannii was the causative agent for 6 HAIs (4 BSIs, 1 VAP, and 1 UTI). Carbapenem-resistant Pseudomonas aeruginosa was the cause of 2 HAIs (1 BSI and 1 VAP).
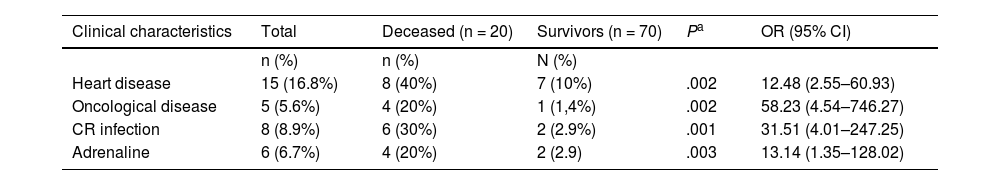
In the bivariate analysis, the following factors were associated with mortality: age less than or equal to 12 months, presence of comorbidity, congenital disease, heart disease, neoplasia, recurrent VAP, septic shock, use of noradrenaline and/or adrenaline, infection by CR organism and having 2 or more HAIs (Table 1). In the multiple logistic regression analysis, heart disease, neoplasia, infection by a CR organism and adrenaline use continued to be associated with mortality (Table 4).
Variables associated with death from sepsis.
| Clinical characteristics | Total | Deceased (n = 20) | Survivors (n = 70) | Pa | OR (95% CI) |
|---|---|---|---|---|---|
| n (%) | n (%) | N (%) | |||
| Heart disease | 15 (16.8%) | 8 (40%) | 7 (10%) | .002 | 12.48 (2.55–60.93) |
| Oncological disease | 5 (5.6%) | 4 (20%) | 1 (1,4%) | .002 | 58.23 (4.54–746.27) |
| CR infection | 8 (8.9%) | 6 (30%) | 2 (2.9%) | .001 | 31.51 (4.01–247.25) |
| Adrenaline | 6 (6.7%) | 4 (20%) | 2 (2.9) | .003 | 13.14 (1.35–128.02) |
CR, carbapenem-resistant.
In our study, the most common HAI among children with sepsis was VAP, followed by BSI and UTI. Most studies conducted in children have found that BSI is the most common infection in this age group,3,12,13 although one descriptive analysis of the epidemiology of HAIs conducted in a PICU in the state of Goiás in Brazil found that the most frequent type was VAP, followed by BSI and UTI.14 Hatachi et al.4 analysed HAIs in the PICU setting and, in line with our study, found no significant differences in the association between different HAIs and mortality. Thus, there appears to be variation in the source of hospital-acquired sepsis and its association with mortality according to study location. Based on our findings, VAP is still a concern in our hospital, as well as in other PICUs in Brazil and worldwide.
As regards the isolated microorganisms, the most frequent were gram-negative bacilli, followed by coagulase-negative Staphylococcus spp. In a multicentre study conducted in Europe, Zingg et al3 found similar results. A study in Brazil found a predominance of Staphylococcus aureus in the PICU setting, followed by Enterobacter spp.15 Another study conducted in data from the National Nosocomial Infections Surveillance System of the United States also found a higher prevalence of Staphylococcus aureus in paediatric patients.16 The authors of a systematic review of evidence of children with sepsis who died reported a prevalence of gram-negative bacilli of 41% in developed countries and 14% in developing countries.7
Considering the 3 types of HAI under study, coagulase-negative Staphylococcus was the most frequent aetiological agent of BSIs, which was consistent with the findings of a study conducted in China that reported a proportion of 44.4% of BSI cases caused by this pathogen.17 In Brazil, in a study conducted in a PICU in the state of São Paulo, gram-positive bacteria were isolated in 72% of BSI cases.18 Other authors have described a higher prevalence of Enterococcus faecalis in children in the United States.16
Although an aetiological agent was only isolated in 17.6% cases of VAP, other authors have also reported low rates of microbiologically-confirmed VAP ranging from 24.4% to 54% of total cases.16,19 The most frequently identified aetiological agent of VAP in our study was Pseudomonas aeruginosa. A study in India analysed the aetiology of VAP in PICUs and found a higher prevalence of nonfermenting gram-negative bacilli, most frequently Acinetobacter baumannii and followed by Pseudomonas aeruginosa19; another study conducted in the United States found that the highest frequency corresponded to Staphylococcus aureus, also followed by Pseudomonas aeruginosa.16
In the group of UTI cases we analysed, the most common microorganism was Klebsiella pneumoniae. Lake et al.20 examined data for children in the United States between 2011 and 2014 and reported a predominance of Escherichia coli, followed by Pseudomonas aeruginosa and Klebsiella pneumoniae. In a study conducted in a PICU in India, Samraj et al.21 found the highest frequency for the Candida fungus, followed by Escherichia coli. In the adult population, Klebsiella pneumoniae was the second most commonly found pathogen in UTIs associated with indwelling urinary catheters, with a high prevalence of antimicrobial resistance.22,23 Another study conducted in Spain that included 104 PICU admissions over a 1-year period concluded that only 6 children had UTIs, 4 caused by Escherichia coli and 2 by Candida albicans.24 A Brazilian study conducted in an adult ICU in the state of Minas Gerais between 2009 and 2010 found a prevalence of UTI of 4.3% with a predominance of Candida albicans, followed by Pseudomonas aeruginosa and Enterobacter aerogenes.25
In our study, we analysed factors associated with mortality in children with HAIs and sepsis. A Brazilian epidemiological study that included children and adults found an overall mortality by sepsis in the ICU of 64.5%,9 higher than the rate we found. The mortality due to hospital-acquired sepsis was 22.2%. A systematic review and meta-analysis published by Tan et al.7 evaluated 94 studies on mortality due to severe sepsis and septic shock in countries worldwide with representation of every continent and found a pooled mortality of 24.7%, similar to the mortality in our cohort. In a study of PICUs in South America, sepsis-related mortality was 14.3% and increased to 23.1% in the case of septic shock.26 Another analysis showed that the presence of septic shock was associated with a six-fold increase in the risk of death in the PICU.27 Our study showed that the mortality due to sepsis in cases who progressed to septic shock was 60%. In the bivariate analysis, the presence of septic shock and the use of noradrenaline or adrenaline were associated with mortality; the latter association remained significant in the multiple regression analysis.
Death from sepsis was associated with age less than 12 months. Ruth et al.,28 who analysed data from a PICU in the United States, found that age less than 1 year was associated with sepsis-related death. Jaramillo-Bustamante et al.27 found a three-fold increase in the probability of death from sepsis in children aged less than 2 years in Colombia.
The presence of comorbidities was significantly associated with death in our sample, and similar results were published by Schlapbach et al.,29 who reported a two-fold increase in risk of death in children with chronic disease. Souza et al.26 analysed sepsis in PICUs in 5 countries in South America and reported a statistically significant association between comorbidities and mortality, concluding that the diseases present in nonsurvivors were most frequently congenital or cardiovascular.
Ruth et al.28 also found an association between underlying cardiovascular and oncological disease and an almost two-fold increase in the probability of death due to sepsis in children, an association that remained significant in the multiple regression analysis in our study.
In the SPROUT study, Weiss et al.30 analysed data corresponding to children in 26 countries on every continent and found no significant association between comorbidities or age and death from sepsis; however, the mortality was higher in younger children with oncological, renal or immunodeficiency diseases.
Recurrent VAP was associated with mortality, which differed from the study by Combes et al.31 in France, who did not find a significant difference in mortality in adult individuals with one versus more episodes of VAP. However, variables such as age and severity scores were associated with mortality in these patients. Another study showed an increase in mortality in adult patients with more than one episode of VAP, but the difference was not statistically significant.32
The association between mortality and infection by carbapenem-resistant bacteria remained significant in the multiple regression analysis, in agreement with the previous the literature. A report issued by the Centers for Disease Control and Prevention33 of the United States stated that more than half of bloodstream infections caused by CR bacteria resulted in death. Alvares et al.34 analysed mortality in children with sepsis due to CR bacteria in Brazil and found that 56.4% died in the CR group compared to 4.65% in the non-CR group (P < .0001). Another study conducted in the United States also found a six-fold increase in the risk of death in children with infection by CR bacteria.35
The presence of two or more HAIs was associated with death from sepsis, similar to what has been reported in adults with multiple sites of infection and higher mortality due to sepsis.36
Although lactate levels were not statistically associated with mortality, previous studies have found an association between an increase in lactate and mortality due to sepsis in children. A cohort study in the United States concluded that an early lactate level greater than 4 mmol/L is associated with a three-fold increase in the probability of death.37 Jaiswal et al.38 found significantly higher lactate levels among nonsurvivors and reported that the lactate level 6 h after diagnosis is an important predictor of mortality.
The level of C-reactive protein (CRP) was also not associated with death. A retrospective study of children in Israel found a statistically significant association between CRP levels of 30 mg/dL or greater and mortality; the authors used the highest recorded CRP level of the patient during the hospital stay in the analysis.39 A Brazilian study that analysed CRP levels in adult patients with sepsis in the ICU found significantly higher levels in those who died. The test was a good predictor of mortality for that population, but the authors performed serial measurements of CRP.40 The associations of increased CRP and lactate levels with death found in these two studies may be explained by the performance of testing closer to the day of death. In our study, we analysed the association with early CRP levels, measured soon after the diagnosis of sepsis, along with lactate levels.
This study has some limitations. It had a retrospective design, was conducted in a single centre study, and the number of patients was small due to the few beds in the participating PICU, which may have hindered the statistical power in inference analyses. Our analysis of sepsis secondary to other HAIs, such as surgical infection, meningitis, diarrhoea or skin infection, might have also hindered it. In addition, the patients were not stratified by severity, and we did not evaluate whether the initial empirical antibiotic treatment of all the HAIs was appropriate for the microorganism isolated in the culture. Factors such as these may have been a source of bias in the results.
On the other hand, this study contributes to increasing our knowledge about important causes of mortality in the PICU. The results of our study can be added to the data of other university hospitals25,26,34 that manage complex patients with severe illnesses. In addition, in our hospital, the frequent changes in care teams, including many students, have been a challenge to improve training in preventive measures against infection and microorganism transmission. At this point, CR bacteria are prevalent in our PICU. We want to highlight this issue, as it reflects the considerable challenge of controlling the spread of multidrug-resistant bacteria, the ensuing difficulty in the treatment of these infections and the high associated mortality.
ConclusionHospital-acquired sepsis had a high fatality rate in the PICU in our hospital. The outcomes were poorer in the youngest patients, patients who required vasopressor drugs, patients with heart disease or malignant tumours and patients with infections caused by carbapenem-resistant bacteria.
FundingThis research project did not receive specific financial support from funding agencies in the public, private or not-for-profit sectors.
Conflict of interestsThe authors have no conflicts of interest to declare.