Acute liver failure (ALF) is a multisystem disease with severe impairment of liver function of acute onset. The Paediatric End-stage Liver Disease (PELD) score is used as a predictor of mortality in chronic liver disease, however experience is limited in ALF.
ObjectivesTo evaluate the aetiology and outcomes of children with ALF in a Children's Liver Transplant Centre, and to investigate the validity of PELD as a prognostic indicator.
Patients and methodsA retrospective study was conducted on patients diagnosed with ALF in our hospital from 2000 to 2013 using the criteria of the Paediatric ALF Study Group.
ResultsThe study included 49 patients with an age range 0–14years. The most frequent aetiologies were: indeterminate (36.7%) and metabolic (26.5%). Liver transplant (LT) was required by 42.8%, and there were 16.3% deaths. Patients with higher levels of bilirubin, INR, or encephalopathy were more likely to require a liver transplant, yielding an OR for INR 1.93. A cut-off of 27 in the PELD score according to the ROC curve showed a sensitivity of 86% and a specificity of 85%, predicting a worse outcome (AUC: 0.90; P<.001). The survival of patients with ALF without transplantation seems more likely in those who have low values of PELD and absence of encephalopathy, with a RR of 0.326.
ConclusionsALF patients with a high PELD score and the presence of encephalopathy had worse outcomes. The PELD score could be a useful tool to establish the optimum time for inclusion in the transplant list, however further studies are still needed.
El fallo hepático agudo (FHA) es una enfermedad multisistémica con afectación severa de la función hepática de aparición brusca. La puntuación Pediatric End-stage Liver Disease (PELD) es un predictor de mortalidad en hepatopatías crónicas, siendo la experiencia en FHA limitada.
ObjetivosEvaluar las características etiológicas y la evolución de niños con FHA en un centro con trasplante hepático (TH) infantil e investigar la validez del PELD como indicador pronóstico.
Pacientes y métodosEstudio retrospectivo de pacientes con FHA en nuestro centro de 2000 a 2013 según criterios del grupo de trabajo de FHA.
ResultadosSe reclutaron 49 pacientes (0-14años). Las etiologías más frecuentes fueron la indeterminada (36,7%) y la metabólica (26,5%). Los pacientes que requirieron TH fueron el 42,8%, y el 16,3% fallecieron. Los pacientes con cifras elevadas de bilirrubina, INR o que desarrollaron encefalopatía tuvieron más probabilidades de presentar una evolución tórpida, obteniéndose una OR para el INR de 1,93. Un punto de corte de 27 en el PELD según la curva ROC mostró una sensibilidad del 86% y una especificidad del 85% de presentar evolución desfavorable (ABC: 0,90; p<0,001). La supervivencia de FHA sin necesidad de TH fue más probable en aquellos con valores de PELD bajos y que no desarrollaron encefalopatía, con un RR de 0,326.
ConclusionesLos pacientes con FHA que presentaron un PELD elevado junto con encefalopatía tuvieron peor evolución. El valor del PELD puede ayudar a establecer el momento óptimo para inclusión en lista de TH; sin embargo son necesarios estudios a mayor escala.
Paediatric acute liver failure (ALF) is a multisystem disorder that gives rise to severe liver failure within days or weeks, manifests with or without encephalopathy, and occurs in children with no pre-existing chronic liver disease. This disease carries a high mortality risk or requires liver transplantation in 70% of cases.1–3
The aetiology of ALF varies by age group and geographical area. In Europe and North America, metabolic disorders and viral infections are the most frequent causes of ALF in newborns. Viral and autoimmune diseases and poisoning are the most frequent causes in older age groups.4–6 However, there is a considerable number of cases (between 40% and 50%) where the aetiology cannot be determined.7,8 Although the actual incidence of paediatric ALF is unknown, it is estimated that it is the reason for 10% to 15% of all liver transplants performed in children.1,6
Supportive measures in intensive care units, treatments for specific aetiologies and all the advances made in liver transplantation, including surgical techniques, immunosuppression and post-transplant care, have increased survival from 60% to 80%.9,10
Despite all these improvements, survival without transplantation is rare and depends on several factors, chief of which are the aetiology of ALF and the age of the patient.11 Thus, the prognosis is bleak in cases of indeterminate aetiology and patients aged less than 2 years. The key to the management of ALF is to identify patients that will recover spontaneously or with disease-specific treatment without the need for transplantation, compared to those with irreversible ALF who are therefore eligible for urgent transplantation.4,12,13
Various prognostic scoring systems have been proposed for the adults, such as the King's College Hospital criteria or the Clichy criteria, which are widely used but have not been validated for use in the paediatric age group.14–17 There are no universal criteria to determine the indication of urgent liver transplantation in paediatric ALF save for cases of acetaminophen toxicity and Wilson disease, for which specific scoring systems have been developed.12,13,18 At present, the international normalised ratio (INR) and factor V are the best predictors of mortality without transplantation in paediatric ALF.
Due to the limitations noted above, we need to develop additional and more versatile prognostic tools for the purpose of determining candidacy for liver transplantation in patients with ALF as reliably as possible. Along these lines, the Pediatric End-Stage Liver Disease (PELD) for children aged less than 12 years, and the similarly named score for patients aged more than 12 years (MELD) were developed for the purpose of determining priority for transplantation in patients with chronic liver disease in the transplant list. Studies have been conducted to explore its validity as a prognostic score in patients with ALF, with excellent results.1,4,10
The aim of our study was to analyse the aetiological characteristics and prognostic factors of ALF in Spain and assess the usefulness of the PELD score as a prognostic indicator (of mortality or the need for liver transplantation) in paediatric ALF for the purpose of determining when patients ought to be placed in the transplant list.
Patients and methodsWe conducted a retrospective study in which we reviewed the cases of all patients aged less than 14 years admitted to our hospital (a liver transplantation referral centre) with a diagnosis of ALF between January 2000 and December 2013.
Following the criteria of the Paediatric Acute Liver Failure working group, we defined ALF biochemical evidence of liver injury and coagulopathy not corrected by vitamin K in the presence of an international normalised ratio (INR) greater than 1.5 in patients with hepatic encephalopathy, or an INR greater than 2 regardless of the presence or absence of hepatic encephalopathy, in patients with no known evidence of chronic liver disease.10,11,19 We excluded patients with severe liver failure in the context of multiple organ failure secondary to known systemic diseases.
We collected data on demographic variables (age, sex, ethnicity and geographical origin) and aetiology. The blood chemistry tests performed were: liver transaminases, bilirubin, ammonia and coagulation factors (INR, prothrombin time [PT] and factor v). We analysed data on the development of coagulopathy, the grade of hepatic encephalopathy (based on electroencephalographic features or the West Haven criteria) and potential complications (infectious, haemorrhagic, respiratory and renal). When it came to the indication for liver transplantation, we applied the King's College criteria for ALF. In cases of acetaminophen-induced ALF, we applied the specific criteria for this aetiology (arterial blood pH<7.30 irrespective of encephalopathy grade or grade iii–iv encephalopathy combined with a PT>100s and serum creatinine>3.2mg/dL). For all other aetiologies, we applied the King's College general criteria of a PT of more than 100s or any three of the following irrespective of the grade of encephalopathy: age less than 10 years, aetiology (non-A/non-B hepatitis, Wilson disease or drug-induced), duration of jaundice to clinical onset of encephalopathy greater than 7 days, PT greater than 50s or serum bilirubin greater than 18mg/dL. We also took into account other factors, such as ventilator dependence or the need for renal replacement therapy, in line with the recommendations of the United Network for Organ Sharing (UNOS).
We calculated the PELD/MELD scores using the values of laboratory tests (albumin, bilirubin and INR) and the height and age of the patient. We used the PELD score in patients aged less than 12 years and the MELD score in older patients. Both scores were calculated at the time of hospital admission using an online calculator (https://optn.transplant.hrsa.gov/resources/allocation-calculators/peld-calculator). We did not perform repeated PELD/MELD calculations due to the potential for biased results, since many patients who required invasive procedures during their stay received fresh frozen plasma transfusions. We divided the sample into two groups based on the outcome of the hospitalisation. The favourable outcome group included patients in who ALF resolved spontaneously or with disease-specific treatment. The unfavourable outcome group included patients that required urgent liver transplantation or died without undergoing transplantation within 16 weeks from admission.
We performed a descriptive analysis, summarising continuous variables (age, bilirubin, INR, prothrombin activity, ammonia and PELD score) as medians and confidence intervals, and categorical variables (sex, aetiology, type of ALF, haemorrhage, need for renal replacement therapy [RRT] and encephalopathy) as absolute frequencies and percentages. We compared quantitative variables in the two outcome groups using the Student's t test and qualitative variables using the χ2 test. We performed logistic regression to develop a model with predictors of mortality or liver transplant, expressing the results as adjusted odds ratios (ORs) with the corresponding 95% confidence intervals. We used the Hosmer–Lemershow test to assess the applicability of the resulting model, and plotted a ROC curve to determine a threshold PELD score for the prediction of unfavourable outcomes. We performed a multivariate analysis with Cox regression to analyse the survival of ALF based on clinical and laboratory data, using the Wald statistic and expressing the results as relative risks (RRs) with 95% confidence intervals. We considered P-values of less than .05 statistically significant. The statistical analysis was performed with the statistics software SPSS version 20.
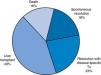
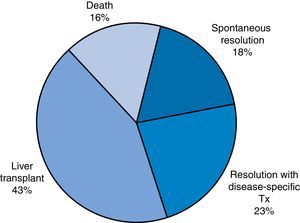
ResultsWe analysed a total of 49 patients who received diagnosis of ALF over a study period of 14 years. We divided the sample based on patient outcomes into the following groups: favourable outcome (spontaneous recovery or resolution with disease-specific treatment; 20 patients [40.8%]) or unfavourable outcome (need of transplantation or death, which finally comprised 29 patients [59.1%]) (Fig. 1).
Table 1 shows the demographic, aetiologic and clinical characteristics of the two patient groups. We found statistically significant differences between the favourable and unfavourable outcome groups when we compared blood chemistry values such bilirubin, INR, ammonia and prothrombin activity. Differences between groups in the presence of complications such as encephalopathy, haemorrhage and need for RRT were also statistically significant.
Demographic, aetiologic and clinical characteristics of the two groups of patients.
| Variable | Favourable outcome (n=20) | Unfavourable outcome (n=29) | P |
|---|---|---|---|
| Sex (%) | |||
| Male | 40 | 51.7 | |
| Female | 60 | 48.3 | |
| Age (years) | 7.17 (± 5.46) | 3.6 (± 4.01) | .01 |
| Aetiology (%) | |||
| Indeterminate | 25 | 44.82 | |
| Metabolic | 30 | 24.13 | |
| Infectious | 10 | 17.2 | |
| Toxic | 15 | 3.4 | |
| Autoimmune | 10 | 6.9 | |
| Haematologic/oncologic | 5 | 3.4 | |
| Vascular | 5 | 0 | |
| Type of acute liver failure (ALF) (%) | |||
| Hyperacute | 50 | 41.4 | |
| Acute | 45 | 44.8 | |
| Subacute | 5 | 13.8 | |
| Encephalopathy (%) | 22.58 | 77.419 | .001 |
| Haemorrhage | 17.391 | 82.608 | .002 |
| RRT | 7.69 | 92.307 | .005 |
| Serum bilirubin (mg/dL) | 8.214 (± 9.565) | 17.08 (± 9.976) | .003 |
| INR | 2.60 (± 1.278) | 4.84 (± 2.12) | <.001 |
| Prothrombin activity (%) | 30.320 (± 10.725) | 15.962 (± 7.626) | <.001 |
| Ammonia (μg/dL) | 139.597 (± 84.519) | 257.379 (± 238.842) | .04 |
| PELD-MELD | 19.90 (± 8.25) | 37.93 (± 10.816) | <.001 |
We developed a logistic regression model to analyse potential predictors of an unfavourable outcome, and found the figures shown in Table 2. The prognostic accuracy of the resulting model was 87.8%, with a sensitivity of 85% and a specificity of 89.7%.
A bilirubin cut-off point of 8.1mg/dL was established by plotting a ROC curve (area under the curve [AUC], 0.793; 95% CI, 0.654–0.932; P=.001), with a sensitivity of 82.8% and a specificity of 75%. As for the INR, the calculated cut-off point was 2.37 (AUC, 0.848; 95% CI, 0.729–0.968; P<.001), with a sensitivity of 86.2% and a specificity of 75%.
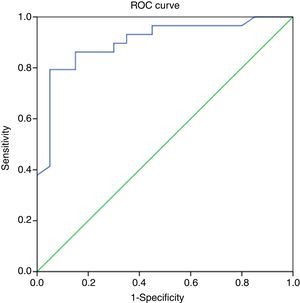
A PELD score cut-off point of 27 in the ROC curve had a specificity of 85% and a sensitivity of 86.2% in predicting a poor outcome of ALF (AUC, 0.9; 95% CI, 0.81–0.99; P<.001), with a Youden index of 0.712, which is indicative of a high specificity (Fig. 2 and Table 3).
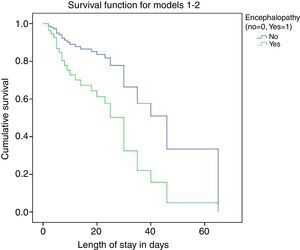
We performed survival analysis by means of Cox regression (Fig. 3), where the dependent variable was unfavourable outcome, defined as death or the need for transplantation during the hospital stay. The independent variables considered in the analysis were: presence of bleeding, hypoglycaemia, infection, intubation, need for RRT, PELD score and encephalopathy. We assessed for potential interactions between variables, which were not statistically significant. The predictors identified in the analysis were the root of the PELD score, with a RR of 2.035 (95% CI, 1.367–3.028) and P<.001, and the absence of encephalopathy, with a RR of 0.364 (95% CI, 0.137–0.973) and P=.04.
DiscussionAcute liver failure is a rare condition but is associated with high mortality, which has been reduced with the development of liver transplantation. The outcomes of ALF range from favourable, with spontaneous recovery or resolution with disease-specific treatment, to the need for transplantation and death. Therefore, we need accurate prognostic tools able to discriminate patients with a poor prognosis for the purpose of selecting patients accurately for early inclusion in the urgent transplant list, optimising the use of the available organs and resources.
The demographic characteristics of the patients in our sample were similar to those described in the previous literature.10 When it came to the aetiology of ALF, metabolic disorders were the most frequently diagnosed cause, corresponding to 30% of the total.20,21 This may be due to our hospital being a referral centre for this type of disease and receiving a large number of immigrant patients, who are a subset in which the incidence is high. We also found a higher frequency of drug-induced ALF in the favourable outcome group compared to the unfavourable outcome group (15% vs 3.4%).
After comparing individual biochemical variables in the two outcome groups and identifying those with significant differences, we developed a logistic regression model that included bilirubin levels, the INR and the presence of encephalopathy as predictors of a poor outcome, with an increase in probability from 59.2% pre test to 89.7% post test. Squires et al.18 obtained similar results.
Since the PELD score includes bilirubin and INR values, it may be useful as a predictor of poor outcome. In our study, a cut-off PELD score of 27 established by means of a ROC curve had a sensitivity of 86.2% and a specificity of 85% in the prediction of unfavourable outcomes. Sanchez and d’Agostino10 established a cut-off PELD score of 33 points with a specificity of 81%. Similarly, Dhiman et al.15 found an optimal cut-off point of 33 for the MELD score, with a specificity of 67.3%. This difference in cut-off points relative to our study may be due to our hospital being a referral hospital for paediatric liver transplantation, as patients considered eligible for transplantation may be referred to us at an early stage.
We performed survival analysis to investigate the presence of complications such as haemorrhage, encephalopathy, infection or the need for renal replacement therapies along with the PELD score, previously described as being significantly associated with outcome, to determine their role as predictors of poor outcomes. The only variables that were significant predictors for a poor outcome were the PELD score and the presence of encephalopathy. The latter is widely recognised in the literature as a predictor of poor outcome. However, when it comes to the PELD score, we recommend a cut-off point of 27 for the purpose of indicating urgent placement in the liver transplant list.
ConclusionsThe PELD score is a tool validated for determining eligibility for liver transplantation in patients with chronic liver disease that includes growth failure, a factor that does not apply to cases of ALF, and serum albumin levels, which have not been found to be significantly associated with ALF.
On the other hand, our results suggest that serum bilirubin levels and the INR can predict poor outcome. The PELD score is calculated using both variables, so (considering the limitations of a complex diagnostic process at young ages) it can be a useful too for predicting the need for transplantation in patients without encephalopathy or mild encephalopathy.
The presence of encephalopathy and the PELD score can be useful factors to include in the comprehensive evaluation of patients with ALF for the purpose of predicting potential outcomes and determining the indication for placement in the liver transplant list, although it is important to be aware of their limitations, as the PELD score was developed for assessment of chronic liver disease, and be adaptive and resourceful in the assessment of these patients. Based on the evidence presented here, further studies are required.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gilbert Pérez JJ, Jordano Moreno B, Rodríguez Salas M. Etiología, resultados e indicadores pronósticos del fallo hepático agudo pediátrico. An Pediatr (Barc). 2018;88:63–68.