As is well known, the standardization by calibration traceable to isotope dilution mass spectrometry (IDMS) of creatinine (Cr) measurement was approved in 2000 with the aim of reducing variability in the results, giving rise to Cr values that were 15%–20% lower.1 This prompted the publication of new reference values for Cr and the modification of the equations used to estimate the glomerular filtration rate (GFR) and for staging chronic kidney disease (CKD) in children, which has significant repercussions.2–5
Recently, Lao et al. (20226 reported that, despite this standardization, there is still significant variability in the obtained results depending on the method used by clinical laboratories. Setting the enzymatic method as the reference, although it is used less frequently due to its higher cost, Jaffe’s colorimetric method exhibits substantial variability, especially in specimens with low concentrations of Cr measured with some of the available commercial assay kits.
By chance, at the time that the Abbott method was introduced in the laboratory of our hospital to replace the Beckman method, we noticed substantial changes in serum creatinine (SCr) measurements in several hospitalized infants that could not be explained by the course of disease and/or the received treatment. Reviewing the sample traceability data, the laboratory analysts concluded that the differences were due to the use of different commercial kits, despite the fact that both were used for the IDMS-standardized Jaffe reaction.
This led us to perform a study to compare the SCr results obtained with the IDMS-standardized Beckman Jaffe or Abbot Jaffe methods with the results obtained with the Abbott enzymatic method.
We started by using 175 serum samples of children aged 15 days to 12 years with SCr values of 0.8 mg/dL or less measured by the Abbott enzymatic method and comparing these values with the values obtained by the IDMS-standardized Abbot and Beckman Jaffe methods. Secondly, we compared the results obtained with the Abbott enzymatic versus Jaffe methods in 819 pediatric samples (0–14 years) with SCr concentrations of 0.8 mg/dL or lower.
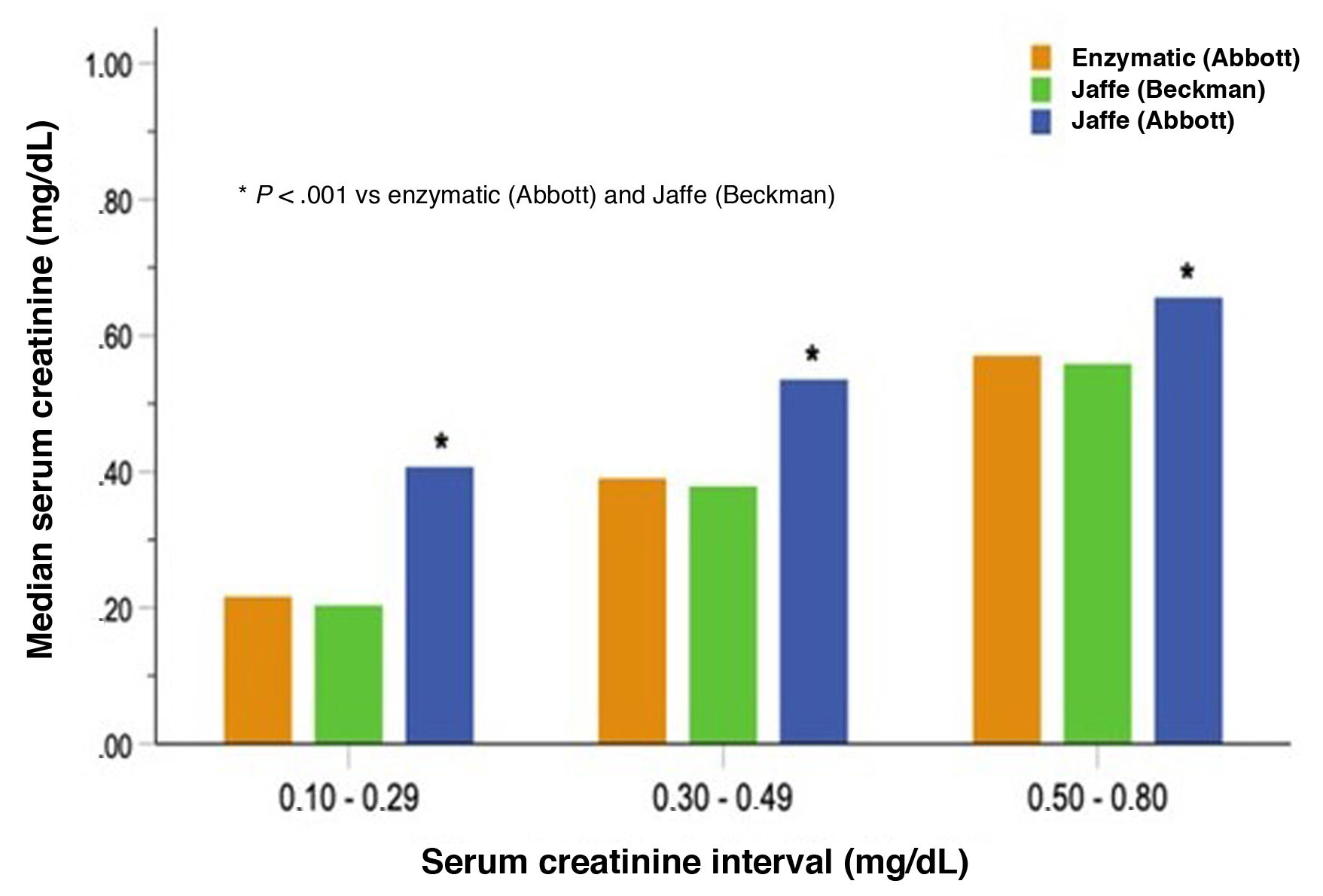
As shown in Fig. 1, the mean SCr values measured by the Abbott Jaffe method were significantly higher (P < .001) than those measured by the Beckman Jaffe and Abbott enzymatic methods, which were similar. The differences were even greater for samples with lower SCr concentrations (< 0.3 mg/dL), with results up to 50% higher with the Abbott Jaffe method (P < .001).
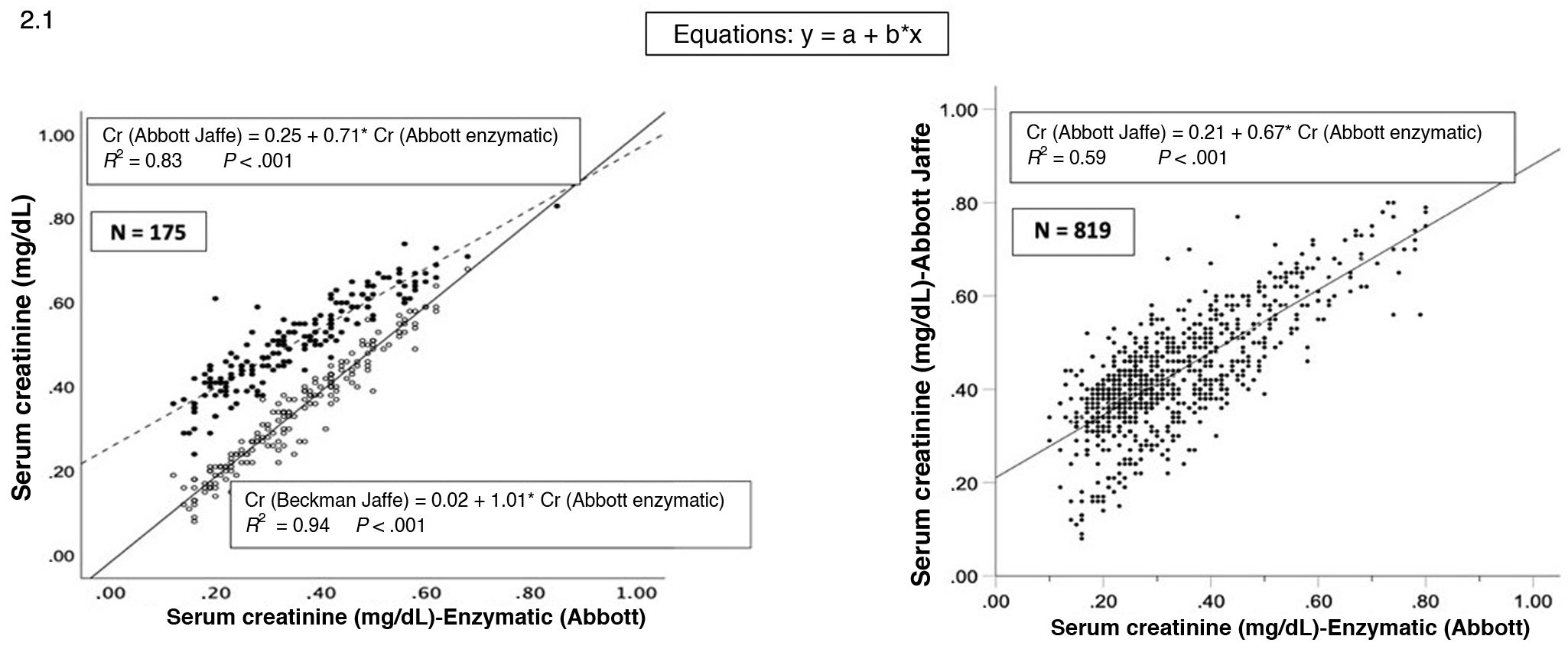
The linear regression analysis of the values obtained from with the Beckman Jaffe versus the Abbott enzymatic method and with the Abbott Jaffe versus the Abbott enzymatic method corroborated these findings (Fig. 2.1). Fig. 2.2 presents the Bland Altman plots for the agreement between the Abbott enzymatic method and the Beckman Jaffe method.
(2.1) Linear regression analysis of serum creatinine values measured by the Beckman Jaffe vs Abbott enzymatic methods and Abbott Jaffe vs Abbott enzymatic methods. (2.2) Brand-Altman analysis for agreement between the compared serum creatinine measurement methods. The mean difference neared 0.0 (perfect agreement) for the enzymatic (Abbott)-Jaffe (Beckman) methods (a) with greater spread for the enzymatic (Abbott)-Jaffe (Abbott) methods (b).
Our findings, in agreement with those reported by Lao, corroborate that standardization to IDMS does not guarantee homogeneity in SCr measurement, chiefly with the Abbott Jaffe method, which overestimates values, especially in the case of low concentrations.6 This can have important consequences in the identification and staging of neonates and infants with CKD.3–5 Therefore, it seems reasonable to recommend the use of the enzymatic method in pediatric samples in clinical laboratories that use the Abbott Jaffe assay.
Last of all, with the aim of knowing the creatinine measurement methods and commercial kits used by hospitals in Spain, we created an online survey and sent it via email to the 45 pediatric nephrology units. The results (response rate, 42/45) revealed that most laboratories in these hospitals used the IDMS-standardized Jaffe method (87%); most frequently with the Roche assay kit (40%) followed by the Abbott (26%), Siemens (20%) and Beckman (14%) kits, with some reporting that they were going to switch to the Abbott assay in the near future.
We believe that it is crucial for pediatric nephrologists to know the method that is used by the hospital for measurement of SCr, and, in the case it is the Abbott Jaffe method, to request the use of the enzymatic method for pediatric serum samples.
The study was approved by the Ethics Committee of the hospital (2025/007).
Meeting presentation: Susana Ferrando Monleón presented this work as a brief communication titled Variability in creatinine serum determination even after IDMS standardization at the VII Hispanic-Portuguese Congress of Pediatric Nephrology; May 18 and 19, 2023; Nova Medical School, Lisbon, Portugal.