Patients with protein metabolism disorders (PMDs) require a diet with strict protein control that can affect their growth and development. The aim of the study was to assess nutritional status and growth in patients with PMDs undergoing dietary treatment.
Patients and methodsProspective observational case-control study in 63 patients with PMDs and 63 healthy controls matched for age and sex. We collected data for anthropometric variables (weight, height, BMI, tricipital and subscapular skinfolds, arm and waist circumference) and calculated the corresponding z scores. We also estimated the body fat mass and classified patients into nutritional status categories.
ResultsThe overall analysis found a lower height z score in patients with PMDs and an equal proportion of overweight and obesity with respect to controls (30.2%). When we divided patients with PMDs into 2 groups (phenylketonuria and other aminoacidopathies), we found that patients with phenylketonuria had a height that was similar to the height of controls and significantly higher compared to patients with other aminoacidopathies. When it came to nutritional status, the prevalence of overweight and obesity was greater in the phenylketonuria group (45.5%), while underweight and short stature were more frequent in the group with other aminoacidopathies.
ConclusionNot all patients with PMDs follow the same growth pattern, and their body composition is variable. In our sample, the group of patients with phenylketonuria had an adequate height but also a higher prevalence of overweight and obesity. On the other hand, patients with other aminoacidopathies had a higher prevalence of underweight and lower z scores for height and arm circumference.
Los pacientes con errores innatos del metabolismo de las proteínas (EIMP) requieren una dieta con control proteico estricto que puede condicionar su crecimiento y desarrollo. El objetivo del estudio es valorar el estado nutricional y crecimiento en pacientes con EIMP sometidos a tratamiento dietético.
Pacientes y métodosEstudio observacional prospectivo caso-control en 63 pacientes con EIMP y 63 controles sanos emparejados por edad y sexo. Se obtuvieron datos antropométricos de peso, talla, IMC, pliegues tricipital y subescapular, perímetros del brazo y cintura, y se calcularon sus z-score. Se estimó la grasa corporal y se clasificó el estado nutricional.
ResultadosEl análisis global mostró un z-score de talla inferior en pacientes con EIMP, e igual proporción de sobrepeso y obesidad respecto a controles (30,2%). Al dividir los pacientes con EIMP en dos grupos (fenilcetonuria y otras aminoacidopatías) se observó que los pacientes con fenilcetonuria tuvieron una talla similar a los controles y significativamente superior a los pacientes con otras aminoacidopatías. Al clasificar el estado de nutrición, la prevalencia de sobrepeso y obesidad fue superior en el grupo de fenilcetonuria (45,5%), mientras que los pacientes más desnutridos y con talla baja se encontraron en el grupo de otras aminoacidopatías.
ConclusiónNo todos los pacientes con EIMP siguen un mismo patrón de crecimiento y su composición corporal es variable. En esta muestra, los pacientes con fenilcetonuria tienen una talla adecuada y mayor incidencia de sobrepeso y obesidad. Sin embargo, otras aminoacidopatías presentan mayores tasas de desnutrición y menor z-score de talla y perímetro del brazo.
Protein metabolism disorders (PMDs) belong to the group of inborn errors of intermediary metabolism, caused by genetic defects in enzymes or cofactors involved in protein and amino acid metabolism. This results in an abnormal accumulation of substrates or a deficit of products in affected reactions, which causes the symptoms characteristic of each disorder.1 At present, thanks to newborn screening, it is possible to diagnose PMDs and initiate treatment early on.2
The treatment of PMDs chiefly consists in restricting the intake of natural proteins of high biological value throughout the lifespan, so the lack of natural protein must be compensated through the delivery of synthetic amino acid mixtures for medical use. The goals of dietary management are to prevent the accumulation of toxic products, maintain metabolic stability and ensure optimal growth and development.3 In periods of metabolic stress, for instance, of intercurrent disease or surgical intervention, dietary management must be adjusted to prevent metabolic decompensation, which entails the addition of a minimum amount of protein while maintaining a high energy intake.4,5
The impact of protein restriction on nutritional status is variable and may affect growth and development in paediatric patients. Therefore, periodic monitoring is required to maintain metabolic stability avoid states of malnutrition, be it undernutrition or overnutrition.
In this context, the aim of our study was to assess the nutritional status and growth of paediatric patients with PMDs managed with dietary therapy and determine whether there were differences in relation to the underlying disease.
Patients and methodsWe conducted a prospective observational case-control study in paediatric patients with PMDs managed by the Nutrition and Metabolic Disorder unit of the Hospital Universitario y Politécnico La Fe in Valencia, Spain, certified as a reference unit (CSUR) for these disorders National Health System of Spain. The study was approved by the Ethics Committee of the hospital.
The study included 63 participants aged less than 18 years with a diagnosis of PMD currently under dietary therapy with strict protein restriction and 63 healthy controls matched for sex and age. We excluded patients with mild disease phenotypes (hyperphenylalaninemia with phenylalanine plasma levels of 2 to 6 mg/dL in repeated measurements) that did not require as strict a restriction of natural protein sources and patients with phenylketonuria (PKU) treated with sapropterin dihydrochloride (Kuvan®) for the same reason. All participants were recruited between 2020 and 2022, and we obtained signed informed consent prior to their inclusion in the study. For each patient, we collected data on age, sex and diagnosis as well as anthropometric values for weight, height, body mass index (BMI), triceps skinfold, subscapular skinfold, arm circumference, waist circumference and fat mass obtained through standardised measurement procedures.6 We calculated the z scores for weight, height and BMI based on the World Health Organization (WHO) growth reference data,7 the z scores of the triceps skinfold, subscapular skinfold and arm circumference based on the Frisancho anthropometric standards8 and the waist circumference z score based on the enKid reference values.9 We calculated the body fat percentage with the equation published by Slaughter et al.10 The classification of nutritional status was based on the WHO growth reference data.7
Statistical analysisWe performed a descriptive analysis, calculating means and standard deviations for quantitative variables. Anthropometric measurements were transformed into z-scores for age and sex prior to other calculations. We expressed categorical variables as absolute frequencies. To compare variables in independent groups, we used the Student t test or the Mann-Whitney U-test depending on whether or not the variables analysed followed a normal distribution. For comparison of continuous variables in more than 2 independent groups, we used analysis of variance (ANOVA) or its non-parametric alternative, the Kruskal-Wallis test. We compared qualitative variables by means of the χ2 test. The level of significance was set at α = 0.05 for all tests.
Given the number of study participants and the fact that PKU was the most frequent disease, we divided the sample into 2 groups (PKU and other aminoacidopathies) to achieve an adequate statistical power.
ResultsThe study included a total of 126 participants (63 patients with PMDs and 63 age- and sex-matched controls) with a mean age of 8.04 years (SD, 4.89) and 47.6% of female participants. In the group with PMDs, the most common disease was PKU, which accounted for 52.4% of the cases (Table 1).
Case distribution by type of disease (n = 63).
| n | % | |
|---|---|---|
| [0,1–3]Urea cycle disorders | ||
| Ornithine transcarbamylase deficiency | 5 | 7.9% |
| Argininosuccinic aciduria | 1 | 1.6% |
| CPS1 deficiency | 2 | 3.2% |
| [0,1–3] | ||
| [0,1–3]Disorders of phenylalanine and tyrosine metabolism | ||
| PKU | 33 | 52.4% |
| Tyrosinaemia I | 4 | 6.3% |
| Alkaptonuria | 1 | 1.6% |
| [0,1–3] | ||
| [0,1–3]Classic organic acidaemias | ||
| Propionic acidaemia | 1 | 1.6% |
| Methylmalonic acidaemia | 4 | 6.3% |
| Isovaleric acidaemia | 1 | 1.6% |
| [0,1–3] | ||
| [0,1–3]Cerebral organic acid disorders | ||
| Glutaric aciduria type I | 9 | 14.3% |
| [0,1–3] | ||
| [0,1–3]Disorders of branched-chain amino acid metabolism | ||
| Maple syrup urine disease | 1 | 1.6% |
| Homocystinuria | 1 | 1.6% |
CPS1, carbamoyl-phosphate synthetase 1; PKU, phenylketonuria.
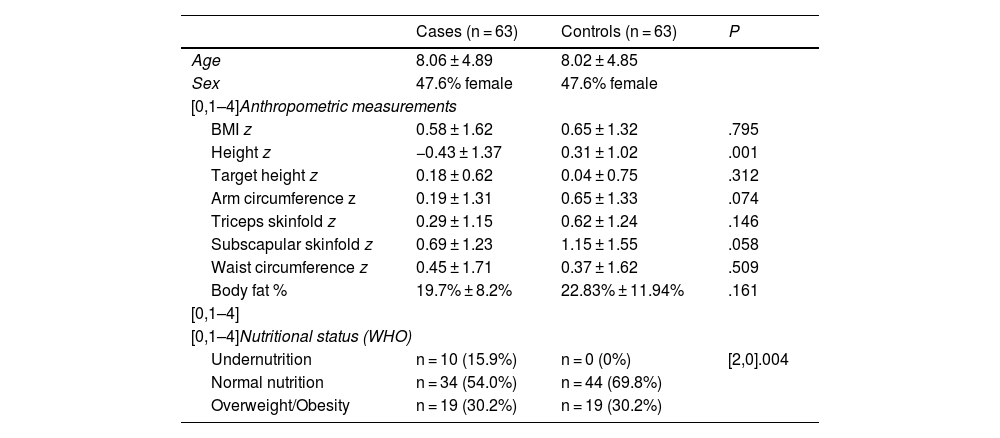
Table 2 presents the anthropometric measurements and nutritional status classification of cases and controls. We found a significantly lower height z score in the group of patients with PMDs. To determine whether this different in height was due to the disease and not a genetic factor, we estimated the target height for both groups (based on data for 75.4% of the sample) and did not find statistically significant differences between groups. There were no statistically significant differences in any other anthropometric values. On the other hand, when we compared nutritional status in the PMD and control groups, we found no differences in the percentage with overweight or obesity, but undernutrition was only detected in patients in the PMD group.
Classification of nutritional status and body composition in cases and controls.
| Cases (n = 63) | Controls (n = 63) | P | |
|---|---|---|---|
| Age | 8.06 ± 4.89 | 8.02 ± 4.85 | |
| Sex | 47.6% female | 47.6% female | |
| [0,1–4]Anthropometric measurements | |||
| BMI z | 0.58 ± 1.62 | 0.65 ± 1.32 | .795 |
| Height z | −0.43 ± 1.37 | 0.31 ± 1.02 | .001 |
| Target height z | 0.18 ± 0.62 | 0.04 ± 0.75 | .312 |
| Arm circumference z | 0.19 ± 1.31 | 0.65 ± 1.33 | .074 |
| Triceps skinfold z | 0.29 ± 1.15 | 0.62 ± 1.24 | .146 |
| Subscapular skinfold z | 0.69 ± 1.23 | 1.15 ± 1.55 | .058 |
| Waist circumference z | 0.45 ± 1.71 | 0.37 ± 1.62 | .509 |
| Body fat % | 19.7% ± 8.2% | 22.83% ± 11.94% | .161 |
| [0,1–4] | |||
| [0,1–4]Nutritional status (WHO) | |||
| Undernutrition | n = 10 (15.9%) | n = 0 (0%) | [2,0].004 |
| Normal nutrition | n = 34 (54.0%) | n = 44 (69.8%) | |
| Overweight/Obesity | n = 19 (30.2%) | n = 19 (30.2%) | |
BMI, body mass index; WHO, World Health Organization; z, z score.
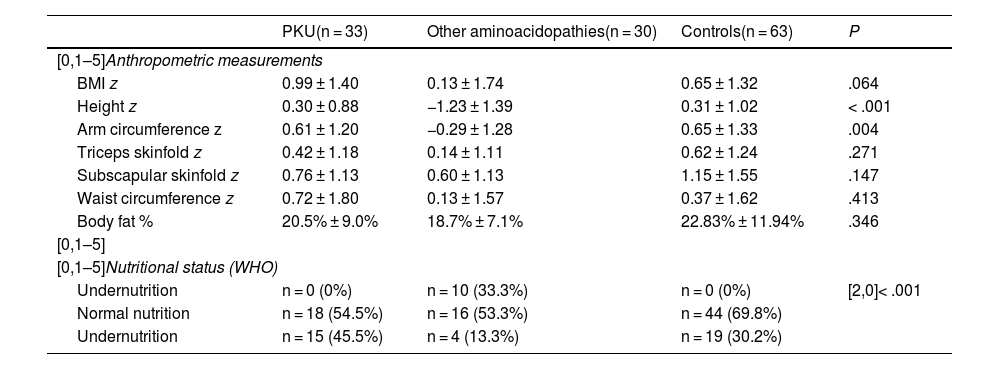
Table 3 presents the anthropometric values and nutritional status classification of participants after dividing the patients with PMDs into two groups (PKU and other aminoacidopathies).
Classification of nutritional status and body composition in cases (categorised by disease group) and controls.
| PKU(n = 33) | Other aminoacidopathies(n = 30) | Controls(n = 63) | P | |
|---|---|---|---|---|
| [0,1–5]Anthropometric measurements | ||||
| BMI z | 0.99 ± 1.40 | 0.13 ± 1.74 | 0.65 ± 1.32 | .064 |
| Height z | 0.30 ± 0.88 | −1.23 ± 1.39 | 0.31 ± 1.02 | < .001 |
| Arm circumference z | 0.61 ± 1.20 | −0.29 ± 1.28 | 0.65 ± 1.33 | .004 |
| Triceps skinfold z | 0.42 ± 1.18 | 0.14 ± 1.11 | 0.62 ± 1.24 | .271 |
| Subscapular skinfold z | 0.76 ± 1.13 | 0.60 ± 1.13 | 1.15 ± 1.55 | .147 |
| Waist circumference z | 0.72 ± 1.80 | 0.13 ± 1.57 | 0.37 ± 1.62 | .413 |
| Body fat % | 20.5% ± 9.0% | 18.7% ± 7.1% | 22.83% ± 11.94% | .346 |
| [0,1–5] | ||||
| [0,1–5]Nutritional status (WHO) | ||||
| Undernutrition | n = 0 (0%) | n = 10 (33.3%) | n = 0 (0%) | [2,0]< .001 |
| Normal nutrition | n = 18 (54.5%) | n = 16 (53.3%) | n = 44 (69.8%) | |
| Undernutrition | n = 15 (45.5%) | n = 4 (13.3%) | n = 19 (30.2%) | |
BMI, body mass index; PKU, phenylketonuria; WHO, World Health Organization; z, z score.
In the comparison of anthropometric measurements among all groups, we found a significantly lower height z score in the group with other aminoacidopathies compared to the control group (P < .001) and to the PKU group (P < .001), with similar values in the control and PKU groups. The remaining anthropometric measurements evinced lower body composition values in the group with other aminoacidopathies compared to the PKU and control groups, with a significant difference in arm circumference. When it came to nutritional status, we found that the proportion of overweight and obese children in the PKU group was significantly greater compared to the control and other aminoacidopathies groups. The latter had the highest proportion of undernutrition among all groups.
DiscussionThis study has shown that patients with PMDs, despite being affected by the same group of diseases and managed with a similar dietary approach, differ in their growth patterns and nutritional status. These findings are relevant, as the management of PMDs requires a diet with strict protein restriction to prevent neurological impairment, but this diet must still allow patients to achieve adequate growth.11,12 Therefore, the achievement of optimal nutritional status and growth is a key objective in their treatment and follow-up.
Overall, comparing cases and controls, we found lower height z scores in patients with PMDs, but similar proportions of overweight and obesity. Previous publications have also reported growth problems in individuals with PMDs,11–13 probably as a result of the restricted intake of natural protein that patients need to maintain throughout the lifespan. In terms of nutritional status, although we found similar results for overweight and obesity in cases and controls in our study, the observed prevalence was lower (30.2%) compared to the prevalence reported by De Castro et al.11 (36.4%). However, the percentage of patients with undernutrition was higher in our sample (15.9%) compared to the study by De Castro (6.5%), although it should be taken into account that the latter study included patients with mild PKU, while our study only included patients with moderate to severe PKU managed with dietary therapy.
In the second stage in the analysis of the sample, we divided the patients into 2 groups according to the type of disease (PKU versus other aminoacidopathies) and observed three distinct patterns: (1) the height of patients with PKU was similar to that of controls and significantly higher compared to the group with other aminoacidopathies; (2) the prevalence of overweight and obesity was higher in the PKU group; (3) undernutrition and short stature were more prevalent in the group with other aminoacidopathies.
With regard to height, most previous studies have reported impaired linear growth in patients with PKU.14–16 However, the study by Belanger-Quintana et al,17 similar to ours, found normal growth in these patients. Based on our results, it is possible that the height of PKU patients is not affected because they are more likely to develop overweight or obesity, and the excess weight may accelerate growth.18 In addition, we found a higher prevalence of short stature in the group with other aminoacidopathies, which was consistent with previous studies,12,19–22 possibly as a result of the greater protein restriction imposed on these patients (for instance, in those with urea cycle disorders), the excessive limitation of an essential amino acid (lysine in patients with glutaric aciduria type 1 or tyrosine in patients with tyrosinaemia type 1) or an imbalance between the intake of an essential amino acid and the total protein intake (for instance, the leucine/protein ratio in methylmalonic or propionic acidaemia).19 There was also an increased prevalence of undernutrition in this group of patients with other aminoacidopathies, so it would be beneficial to monitor nutritional status in these patients more closely to prevent long periods of undernutrition that could affect the final height.
With respect to the remaining anthropometric measurements under study, patients with PKU had higher values compared to the group with other aminoacidopathies, although the differences were only significant for arm circumference. We have not found any studies in the literature that provide data on this parameter for comparison. As regards the body fat percentage, some authors23–25 have reported greater percentages in patients with PKU compared to controls or the reference population, but other studies26–29 have found similar values, as was the case in our sample. Few studies have analysed the body fat percentage in patients with other aminoacidopathies. Some authors have reported high fat mass percentages (of up to 40%) in patients with propionic or methylmalonic acidaemia,19,22 but in our cohort the mean body fat percentage in these patients was 18.7%, similar to the values published by Evans et al.12 and in the control group.
With regard to nutritional status, which we analysed by disease group, the proportion of patients with overweight or obesity was significantly higher in the group with PKU compared to controls and patients with other aminoacidopathies. In the previous literature, the nutritional status results in patients with PKU are heterogeneous,30 although there seems to be a tendency toward excess weight in association with more severe phenotypes, especially in female patients and around adolescence.24,27,31–35 In this regard, since we excluded patients with mild phenotypes from the study, our findings seem to make sense. However, adequate nutritional intervention is still beneficial to prevent or correct excess weight. In this regard, there are studies that suggest that the increased dietary glycaemic index and glycaemic load and of patients with PKU can be chiefly attributed to low-protein products (pasta, rice, biscuits, etc.), which are processed, as opposed to phenylalanine-free complete amino acid mixes.36 Thus, it would be beneficial to carry out a detailed assessment of the quality of the diet in these patients and improve nutrition education concerning the consumption of these products,37 promoting the intake of natural foods that can be consumed freely or in controlled amounts.
It is important to take into account the limitations of this study, which include the variety of diseases included in the group of other aminoacidopathies and the small number of patients who had each of them. However, the total sample size was adequate considering that these are diseases with a very low prevalence. Another limitation was that we did not assess the stage of pubertal development, and there may be variability in both male and female patients during adolescence.
ConclusionAlthough all the diseases analysed in this study are PMDs managed with a dietary therapy with protein restriction, the growth pattern was not consistent in the group of patients with PMDs and there was substantial variation in their body composition.
Patients with PKU achieved adequate heights, similar to controls, but there was a higher frequency of overweight and obesity in this group. On the other hand, undernutrition and poor growth were more frequent in the group of patients with other aminoacidopathies.
These findings reflect the importance of assessing the quality of the diet and routinely monitoring body composition parameters in the follow-up of patients with PMDs to ensure adequate growth and nutritional status.
FundingThis research did not receive any external funding.
Meeting presentation: This study was presented as a poster at the XXIX Congress of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica; April 20–22, 2023; Córdoba, Spain.