Non-invasive mechanical ventilation (NIV) is used in neonatal units to decrease the use of invasive mechanical ventilation.1,2 Our objective was to determine the frequency of use of NIV, its indications and the ventilators, parameters and modalities of ventilation used. Between April and May 2017, we sent an online questionnaire to the neonatal units in Spain that offer higher levels of care, and requested that a representative for the unit respond to the questions based on the actual practices of the unit as opposed to personal preference. We present a descriptive analysis of the data, expressing categorical data as absolute frequencies and percentages, and continuous data as median and interquartile range (IQR).
We contacted 67 units, and 44 participated (response rate of 66%). Ninety-six percent were level III units. Ninety-three percent used some form of NIV.
More than half of the units (56%) used NIV for initial respiratory support in the management of respiratory distress syndrome in preterm newborns delivered before 30 weeks’ gestation. Twenty-nine percent used NIV for first-line respiratory support due to the high risk of failure of continuous positive airway pressure (CPAP) in these immature patients, and 27% used NIV or CPAP to initiate support depending on the clinical condition of the patient or the judgment of the physician in charge. Sixty-two percent (24/39) used NIV during less invasive surfactant administration (LISA).
Table 1 presents the generators and interfaces used in the units, and Fig. 1 the ventilation parameters. Less than half of units in Spain (49%) had the capability to deliver synchronized NIV, and only 3 used this approach routinely for NIV. The most widely used synchronization system was the abdominal capsule of the Infant Flow® (10/20). The Giulia® (flow-synchronized ventilator) is used in 7 units, and neurally-adjusted ventilatory assist (NAVA, a synchronization system based on the electrical activity of the diaphragm) in 3. The use of other synchronization systems was anecdotal. When asked for the reason for rarely or never using synchronization, 65% of respondents replied they did not have the necessary equipment or disposable supplies for it and only 2 respondents argued that the current evidence does not support the superiority of synchronized NIV over non-synchronized NIV in newborns. Only 44% of surveyed neonatal units had an established NIV protocol.
Generators and interfaces used for CPAP and NIV in neonatal units in Spain.
| Generator | nCPAP, n (%) | NIV, n (%) |
|---|---|---|
| Bubble CPAP® | 5/44 (11%) | -------------- |
| Hamilton Medical Aladdine/Arabella® | 8/44 (18%) | -------------- |
| Medical Sorevan CPAP medinCNO®/Medijet® | 7/44 (16%) | 2/41 (5%) |
| Ginevri Medical Technologies Giulia® | 7/44 (16%) | 7/41 (17%) |
| Acutronic Fabian CPAP® | 14/44 (32%) | 11/41(27%) |
| Adaptation of conventional respirator | 25/44 (57%) | 21/41 (51%) |
| Care Fusion Infant Flow® | 28/44 (64%) | 27/41 (66%) |
| Interfaces | nCPAP, n (%) | NIV, n (%) |
|---|---|---|
| Short binasal prongs | 41/44 (93%) | 41/41 (100%) |
| Nasal mask | 41/44 (93%) | 38/41 (93%) |
| Nasopharyngeal or mononasal tube | 8/44 (18%) | 7/41 (17%) |
| RAM cannula | 6/44 (14%) | 4/41 (10%) |
| Oronasal face mask | 5/44 (11%) | 4/41 (10%) |
| Helmet | 3/44 (7%) | -------------- |
The used CPAP and NIV generators varied between units, and the most frequently used was the Care Fusion Infant Flow® generator, followed by conventional ventilators used for invasive ventilation adapted for NIV. Furthermore, 80% of hospitals used different NIV generators in the same unit. The most frequently used interfaces for both NIV and CPAP were short binasal prongs and nasal masks.
CPAP, continuous positive airway pressure; nCPAP, nasal CPAP; NIV, non-invasive ventilation.
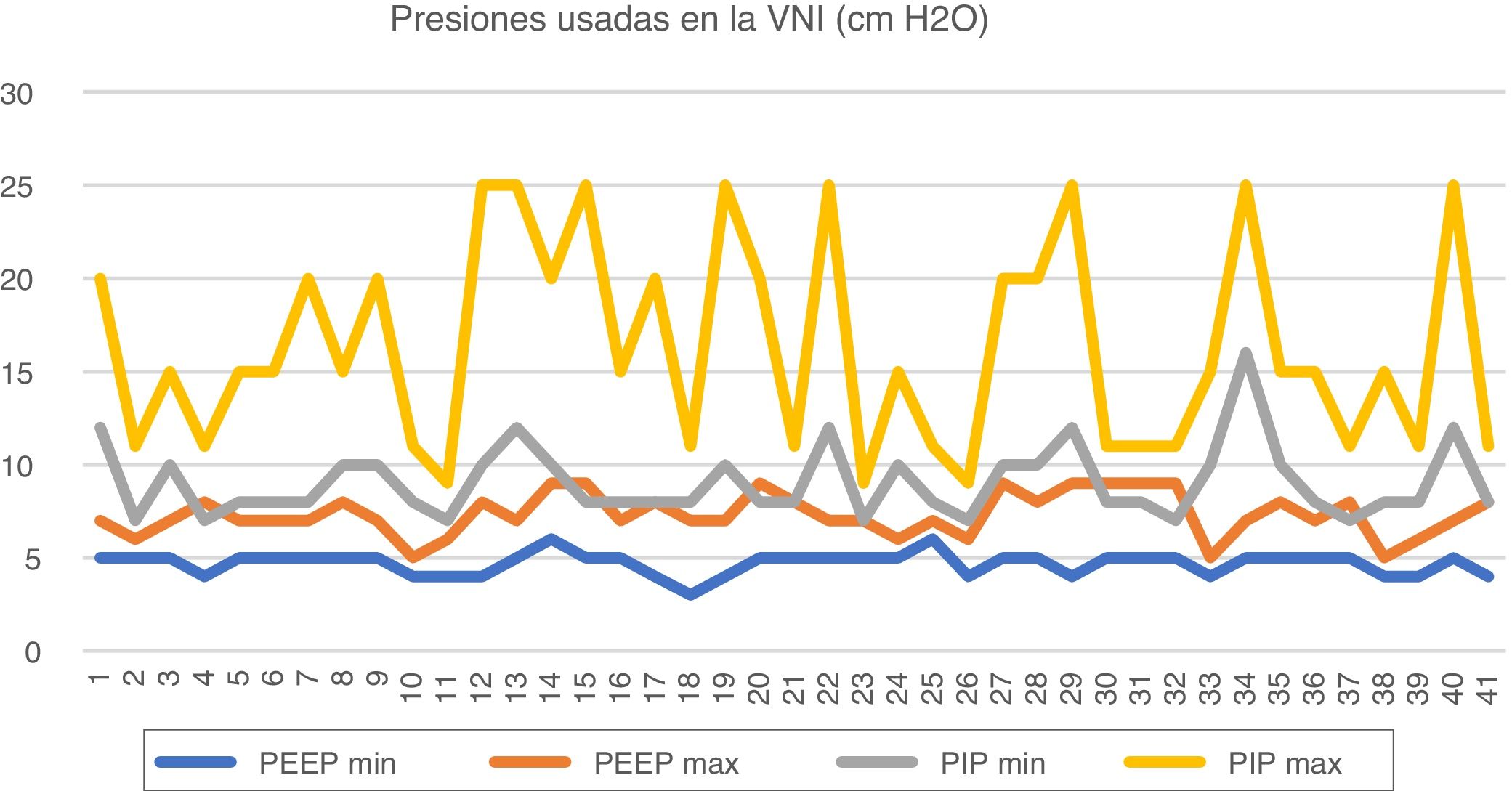
Minimum and maximum parameters used in non-invasive mechanical ventilation.
The minimum and maximum PEEPs used in CPAP are 4 (4–5) cm H2O and 7 (7–8) cm H2O. The minimum and maximum PEEPs used in NIV are 5 (4–5) cm H2O and 7 (7–8) cm H2O. The minimum PIP used in NIV is 8 (8–10) cm H2O and the maximum PIP 15 (11–20) cm H2O. When using NAVA (3 hospitals) the maximum set was the maximum considered acceptable for the given parameter.
Other parameters used are: respiratory rate, minimum 20 bpm (11–30) and maximum 50 bpm (40–60); inspiratory time, minimum 0.3 s (0.3–0.4) and maximum 0.5 s (0.4–0.6).
bpm, Breaths per minute; NIV, non-invasive mechanical ventilation; PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure.
Our findings show that NIV is widely used in neonatal care units in Spain (93%), but that practices are heterogeneous in terms of the systems and parameters used. Although European guidelines3 have concluded that there is not sufficient evidence to recommend NIV for initial respiratory support in RDS, more than half of the surveyed units used it in this way. It was also frequently used during LISA (62%). This technique has become widespread in clinical practice in Spain,4 and since its outcome depends on the maintenance of an adequate work of breathing, this may make NIV the preferred modality of respiratory support during this intervention.
Neonatal NIV clinical trials have been methodologically heterogeneous, which complicates the interpretation of their results, but it is possible that not all NIV modalities have the same benefits or indications.1,5,6 The generator used most frequently in Spanish neonatal units was the Infant Flow®, a variable flow generator for bilevel positive airway pressure (BiPAP) that allows control of the peak inspiratory pressure (PIP) in ventilation delivery. Although it is used in 66% of units, there is no clear evidence of its superiority over CPAP,3,5,6 and it may even be controversial to consider this BiPAP a form of NIV.5 The parameter settings may also vary between facilities, which could be explained by the substantial heterogeneity of published NIV trials. The greatest variability corresponds to the maximum PIP considered acceptable, probably because many different generators are currently in use. Synchronized modalities are rarely used, and most frequently used system is the abdominal capsule of the Infant Flow®, although the need for synchronization in the BiPAP modality is unclear.5
In short, NIV is applied in the usual clinical practice of neonatal units in Spain, even in clinical scenarios for which there is still no consensus, such as the initial respiratory support in the management of RDS and during LISA. The NIV approaches currently implemented vary widely in terms of generators, parameters and synchronization. Although the greatest benefits have been described with synchronised NIV with a ventilator, synchronization is rarely used and BiPAP generators widely used. Further studies are required to establish the benefits and potential differences between NIV modalities. The development of clinical practice guidelines could help standardise the use of the different NIV modalities.
Please cite this article as: Fernández García C, Comuñas Gómez JJ, Montaner Ramón A, Camba Longueira F, Castillo Salinas F. Encuesta nacional sobre el uso de ventilación mecánica no invasiva en las unidades neonatales españolas. An Pediatr (Barc). 2022;97:138–140.